
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
28 please
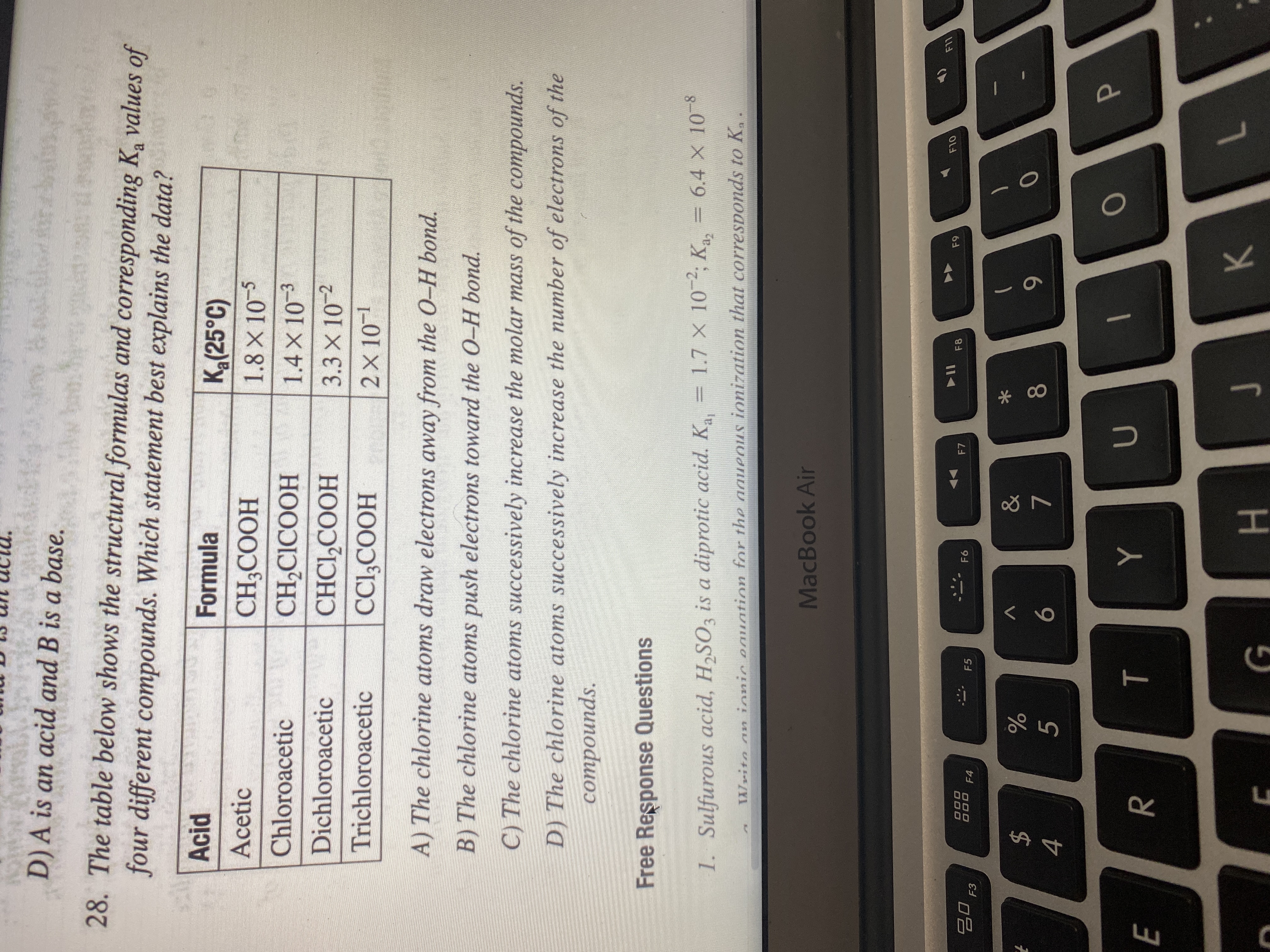
Transcribed Image Text:Free Response Questions
1. Sulfurous acid, H2SO3 is a diprotic acid. K
= 1.7 X 10-2, K = 6.4 x 10-8
6.4 X 10 8
TWrito an ionic eauation for the aaueous ionization that corresponds to K,.
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH,N, Et,0, CH,Cl, 30 min HO,arrow_forwardNeed help with homeworkarrow_forwardCHM 431A Lab 7- 2021SP Given Data page Time Temperatu (min) C1. Boiling temp of water re (°C) 25.4 Initial temperature of water 32.5 Temperature of boiling water 44.9 3. 56.9 C2. Temperature change (AT) 4. 71.3 Mass of beaker 5. 86.6 100.2 Mass of beaker and water 7. 100.6 C3. Mass of water 8. 100.6 100.6 1. 2) 6.arrow_forward
- HO + Drawing N = Qarrow_forwarda W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 30.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 05 106 107 108 109 110 111 112 114 116 118 Ob Sg Bh Hs Mt Ds Uuu Uub 260) (263) (262) (265) (266) (271) (272) (277) (296) (298) (7) 60 61 62 63 64 65 66 67 68 69 70 71 Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 44.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 92 93 94 95 96 97 98 99 100 101 167.3 168.9 173.0 175.0 102 103 U Np Pu Am Cm Bk Cf F Es Fm Md No Lr 238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) sogna pee ou cacau pe busca o cus £(H၁)၁၀- шогеш аз моц моцѕ 01 смоле рәліп əsənу sәне иo pǝseq pod еш әсхә пол t be a part by a padd a test so be aarrow_forward8 - 14 pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY