
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Calculate the change in enthalpy ΔH for 100 kg of material undergoing the following temperature and phase changes. Show your calculations in detail (including the integrations) type them out in word.
- benzene in liquid state heated from 20 °C, 1 atm to vapor at 120 °C, 1 atm. Include in your solution for this problem a diagram of the process path. (piecemeal)
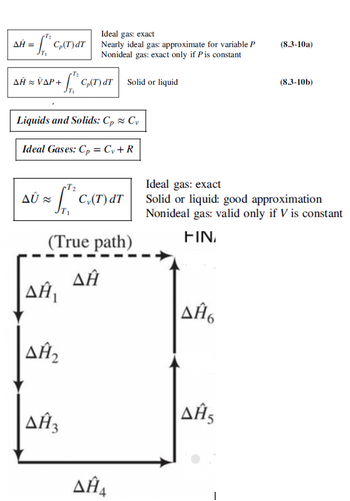
Transcribed Image Text:ΔΗ Ξ
Ideal gas: exact
f GT)ar Nearly ideal gas: approximate for variable P
Τι
Nonideal gas: exact only if P is constant
(8.3-10a)
Τ
ΔΗ ~ ΥΔΡΑ
corsar
Solid or liquid
(8.3-10b)
Liquids and Solids: Cp ~ Cy
Ideal Gases: Cp = C + R
Δύ
T₂
C₁(T) dT
(True path)
ΔΗ,
ΔΗ,
ΔΗ
Ideal gas: exact
Solid or liquid: good approximation
Nonideal gas: valid only if V is constant
FIN
ΔΗ
ΔΗ
ΔΗ,
ΔΗΑ
![Form 1: Cp[kJ/(mol·°C)] or [kJ/(mol·K)] = a + bT + cT² + dT³
Form 2: Cp[kJ/(mol·°C)] or [kJ/(mol·K)] = a + bT + cT−2
Example: (Cp)acetone(g) = 0.07196 + (20.10 × 10-5)T - (12.78 × 10−8)7² + (34.76 × 10-12)73, where T is in °C.
Mol.
Temp.
Range
(Units
Compound
Formula
Wt. State Form Unit
a X 10³ b x 105
cx 108
d x 1012
of T)
Benzene
C6H6
78.11
1
1
°C
126.5
23.4
6-67
g
1
°C
74.06
32.95
-25.20
77.57
0-1200](https://content.bartleby.com/qna-images/question/d47fc2c3-8f4b-4085-b234-bc909be774e3/02c4b26b-9554-4a86-883d-929d9792d4aa/8lo0a6wm_thumbnail.png)
Transcribed Image Text:Form 1: Cp[kJ/(mol·°C)] or [kJ/(mol·K)] = a + bT + cT² + dT³
Form 2: Cp[kJ/(mol·°C)] or [kJ/(mol·K)] = a + bT + cT−2
Example: (Cp)acetone(g) = 0.07196 + (20.10 × 10-5)T - (12.78 × 10−8)7² + (34.76 × 10-12)73, where T is in °C.
Mol.
Temp.
Range
(Units
Compound
Formula
Wt. State Form Unit
a X 10³ b x 105
cx 108
d x 1012
of T)
Benzene
C6H6
78.11
1
1
°C
126.5
23.4
6-67
g
1
°C
74.06
32.95
-25.20
77.57
0-1200
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The