
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN: 9781133939146
Author: Katz, Debora M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Topic Video
Question
What is the force of buoyancy applied to the sphere ball with the diameter of 10
m when 30% of the volume is under water?
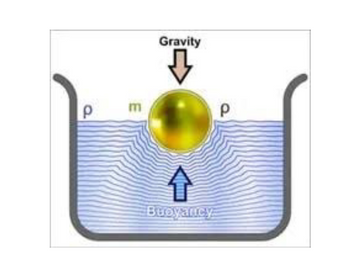
Transcribed Image Text:P
Gravity
Ĵ
Buoyancy
Р
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Check Your Understanding Two objects A and B have the same dimensions identically. A is of a material with a higher thermal expansion coefficient B. If the objects are heated identically, will A feel a greater Stress than B?arrow_forwardFind the number of moles in 2.00L of gas at 35.0C and under 7.41107N/m2 of pressure.arrow_forwardWhat does it mean to say that two systems are in thermal equilibrium?arrow_forward
- A pressure cooker contains water and steam in equilibrium at a pressure greater than atmospheric pressure. How does this greater pressure increase cooking speed?arrow_forwardA high-pressure gas cylinder contains 50.0 L of toxic gas at a pressure of 14107 N/m2 and a temperature of 25.0 . The cylinder is cooled to dry ice temperature ( 78.5 ) to reduce the leak rate and pressure so that it can be safely repaired. (a) What is the final pressure in the tank, assuming a negligible amount of gas leaks while being cooled and that there is no phase change? (b) What is the final pressure if one-tenth of the gas escapes? (c) To what temperature must the tank be cooled to reduce the pressure to 1.00 atm (assuming the gas does not change phase and that there is no leakage during cooling)? (d) Does cooling the tank as in part (c) appear to be a practical solution?arrow_forwardIn state-of-the-art vacuum systems, pressures as low as 1.00 x 10-9 Pa are being attained. Calculate the number of molecules in a 2.00-m³ vessel at this pressure and a temperature of 30.0°C. molecules Need Help? Read It Watch Itarrow_forward
- An infrared heater for a sauna has a surface area of 0.050 m2 and an emissivity of 0.84. What temperature must it run at if the required power is 360 W? Neglect the temperature of the environment.arrow_forwardThe density of water at 0 C is very nearly 1000 kg/m3 (it is actually 999.84 kg/m3), whereas the density of ice at 0 C is 917 kg/m3. Calculate the pressure necessary to keep ice from expanding when it freezes, neglecting the effect such a large pressure would have on the freezing temperature. (This problem gives you only an indication of how large the forces associated with freezing water might be.)arrow_forwardNoting the large stresses that can be caused by thermal expansion, an amateur weapon inventor decides to use it to make a new kind of gun. He plans to jam a bullet against an aluminum rod inside a closed invar tube. When he heats the tube, the rod will expand more than the tube and a very strong force will build up. Then, by a method yet to be determined, he will open the tube in a split second and let the force of the rod launch the bullet at very high speed. What is he overlooking?arrow_forward
- Under what circumstances would you expect a gas to behave significantly differently than predicted by the ideal gas law?arrow_forwardTypical molecular speeds (vrms) are large, even at low temperatures. What is vrms for helium atoms at 5.00 K, less than one degree above helium's liquefaction temperature ?arrow_forwardMost cars have a coolant reservoir to catch radiator fluid that may overflow when the engine is hot. A radiator is made of copper and is filled to its 16.0-L capacity when at 10.0 . What volume of radiator fluid will overflow when the radiator and fluid reach a temperature of 95.0 , given that the fluid's volume coefficient of expansion is =400106/C? (Your answer will be a conservative estimate, as most car radiators have operating temperatures greater than 95.0 ).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning