
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For the molecule below:
a. Calculate the hydrogen deficiency index
b. Label significant absorption bands in the IR spectrum w/the bond repsoinsible
c. List possible funcational groups
d. Draw possible bond line structure for the molecule
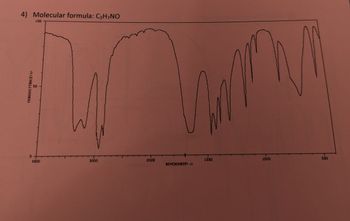
Transcribed Image Text:4) Molecular formula: C3H7NO
TRANSMITTANCE!!
D
LDD
5D
4000
3000
2000
HAVENUMBERI-11
ww
1500
1000
500
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. For following IR spectra: A. Identify the functional groups and bond types present in the molecule. B. Label peaks in the IR spectra below. Benzene LOD D. 3000 2000 1000 5po 4000 HAVENUMB ERI -l TRANSMITTANCEIXIarrow_forwardWhen analyzing an IR spectrum, the most significant peaks usually are: a) Given by stretching vibrations in the fingerprint region b) Caused by bending vibrations at low wavelength values c) Given by isotopes of the most abundant elements d) Caused by stretching vibrations at high wavelength valuesarrow_forward(All spectra are from the AIST database) 1. For following IR spectra: A. Identify the functional groups and bond types present in the molecule. B. Label peaks in the IR spectra below. 2-METHYL-2-BUTENE C5H10 LOD D 4000 3000 200 1500 1000 5 00 HAVENUMB ERI -1l TRANSMITTANCEIarrow_forward
- Could someone help me determine the splitting pattern (multiplicity) for each of the labeled hydrogen and draw each of the peaks please? Thank you :)arrow_forwardFor the molecule below: a. Calculate the hydrogen deficiency index b. Label significant absorption bands in the IR spectrum w/the bond repsoinsible c. List possible funcational groups d. Draw possible bond line structure for the moleculearrow_forward1. BH-/THF 2. H,O,/NAOH Which of the following is the major organic product of the reaction shown above? (A) OH (B) OH (C) (D) НО ОН (E) HOarrow_forward
- For Hydrogen D, could the splitting pattern be more complex such as doublet of doublet or doublet of triplets, etc.? We are not supposed to say multiplet, even though the image is blurry we cannot say multiplet. Could you please go over all the splitting patterns for the different hydrogens. Please also indicate which peaks are relevant in the ir, using the table below for the ir spectra that was given earlier.arrow_forwardQUESTION 3 Why is carbon-hydrogen splitting not a major part of 1H NMR spectra? a. because the carbon-hydrogen coupling constant is so small b. because the energy level used for 1H NMR spectra doesn't effect 13C. c. because the hydrogen hydrogen splitting masks t Od. because 13C is only 1% of the naturally occurring C Oe. because carbon-hydrogen splitting doesn't occur QUESTION.arrow_forwardQuestion 8arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY