
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
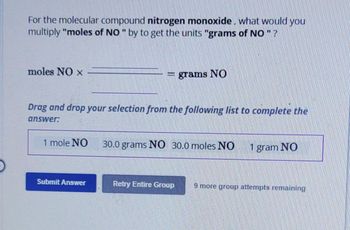
Transcribed Image Text:For the molecular compound nitrogen monoxide, what would you
multiply "moles of NO " by to get the units "grams of NO " ?
moles NO X
1 mole NO
www
Drag and drop your selection from the following list to complete the
answer:
Submit Answer
grams NO
30.0 grams NO 30.0 moles NO
Retry Entire Group
1 gram NO
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many moles of nitrogen atoms are present in 49.2 g of NH4NO3? Provide your answer in units of moles using at least three significant figures. ( Gpt/Ai wrong answer not allowed)arrow_forwardConsider the following unbalanced equation: C2H6(g) + O2(g) → CO2(g) + H2O(g) Which amount requires more oxygen gas, 2.35 mol C2H6 or 30.6 g C2H6? What information do we need to determine which amount of C2H6 requires more oxygen gas? Select ALL correct options. Group of answer choices 2.35 mol C2H6 molar mass of C2H6 balanced chemical equation number of grams present in 1 kilogram 30.6 g C2H6arrow_forwardplease make sure the answer has the correct number of significant digitsarrow_forward
- C5H12(1) + 8 O2 (g) --> 6 H2O (g) + 5 CO2 (g) How many moles of CO2 will be produced by 56.0 grams of C5H12 ? Round your answer to 2 decimal places. Your Answer: Answer unitsarrow_forwardHow many grams of H2SO4 are present in 1.9*1026 formula units of H2SO4 ? Round your answer to 1 decimal place. Your Answer: Answer unitsarrow_forwardThe molecular formula of remdesivir is C27H35N6O8P. How many nitrogen atoms are in 6.4555 moles of remdesivir? Enter your response rounded to 3 significant figures.arrow_forward
- Convert each of the following. Write out the full conversion factor. 5.34 x1027 molecules of sulfur hexafluoride to moles of sulfur hexafluoride. 2. 4.1 moles of sodium carbonate to molecules of sodium carbonate. 3.0.55 moles of sodium nitrate to molecules of sodium nitrate.arrow_forwardSTARTING AMOUNT X Aluminium has a density of 2.70 g/cm³. How many moles of aluminum are in a ADD FACTOR x( ) 35.6 1.32 molecules Al 13.2 cm³ block of the metal substance? 2.70 0.489 g Al/mol 0.100 26.98 g/cm³ = ANSWER 962 g Al 13.2 1 mol Al RESET 5 6.022 × 10²3 cm³arrow_forwardmay you answer and explain this for me please?arrow_forward
- QUESTION 9 How many moles of H20 are in a sample with a mass of 14.9 grams? Report your answer to 3 significant figures. Do not include units on your answer. To report an answer using scientific notation, use the following format Ex 6.02x1023 would be entered as 6.02E23.arrow_forwardUsing the equation below, convert 14.78 grams of water to moles of H2SO4. Please round your answer to two digits after the decimal point and don't forget units and substance! SO3 + H2O --> H2SO4arrow_forward1.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 192. g/mol, is completely in excess oxygen, and the mass of the products carefully measured: product carbon dioxide water 0 mass 2.06 g 0.56 g Use this information to find the molecular formula of X. Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY