
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
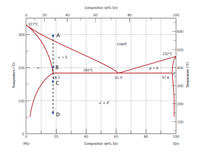
For the lead-tin system:
(a) Determine the proportions of liquid and solid phases for the 40% Sn composition of
the lead-tin system at temperatures 2500?, 200
0?, ??? 1500?.
(b) Draw the development of microstructure along the dashed line at points A,B,C, and
D.
Transcribed Image Text:Composition (at% Sn)
20
40
60
80
100
327°C
H600
300
Liquid
500
232°C
a + L
200
400
183°C
18.3
61.9
97.8
300
100
a + B
200
100
20
40
60
80
100
(Pb)
Composition (wt% Sn)
(Sn)
Temperature (°C)
Temperature (°F)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- According to the following graph, two samples of 1080 steel are cooled from the eutectoid temperature, one at a cooling rate of 250°C/s and the other at a cooling rate of 7.27x10-8 °C/s. Specify the phases obtained and explain their formation from thermodynamic and kinetic perspectives. Also, briefly describe their formation. Draw the microstructure of the phases obtained. Sıcaklık (C) 800 700 600 500 400 300 200 100 0 10 1 T M(başlama) M(% 50) M(% 90) 10 M+O -Otektoid Sıcaklık 10² Zaman (s) % 50 10³ 104 105arrow_forwardRead the question carefully and give me right solution according to the questionarrow_forwardGiven the equilibrium phase diagram (a) below, briefly describe the mechanisms of precipitation hardening/strengthening of an aluminium alloy making reference to the transitions shown in (b) below to X, X to A, X to D, A to B and A to C. а +0 D B Time (a) (b) - anedua Temperaturearrow_forward
- pls immediately help mearrow_forwardA hypothetical eutectic phase diagram for metals A and B shows that cooling a liquid melt with a concentration of 50 wt% A-50 wt% B to just below 625 °C (eutectic isotherm temperature) results in simultaneous formation of alpha and beta phases. The phase diagram also shows that alpha (A rich) and beta (B rich) phases exist at left and right extremes of the phase diagram, respectively. At the eutectic isotherm temperature, the maximum solubility of B in A and A in B is 10 wt% and 5 wt%, respectively. The phase diagram does not have any intermetallic compounds. A new solid alloy is made by melting together 86 gr of a 40 wt% A-60 wt% B alloy with 136 gr of 65 wt% A-35 wt% B alloy followed by cooling the melt to 400 °C. If the new alloy is heated up from 400 °C to just below 625 °C, answer the following questions: a. Calculate the overall alloy composition. b. Determine phases present and calculate their respective masses. Calculate the masses of the primary phase, eutectic phase, and…arrow_forwardConsider the phase diagram below. The three points A, B, and C are at concentrations of 27, 31.9, and 33.8 wt% Ni respectively. The ends of the tie line are at C1 = 25% wt% Ni and C2 = 35 wt% Ni. What are the weight fractions of the alpha phase at A and the L phase at B, as well as the alpha phase/L phase ratio at C? T(°C) 1300-L (liquid) 1200 20 ABC L + a C1 30 S C2 liquidus 40 L + a solidus α (solid). O a. Walpha=0.12; WL=0.41; Walpha/WL = 9.41 O b. Walpha=0.27; WL-0.26; Walpha/WL = 11.11 O c. Walpha=0.15; WL-0.26; Walpha/WL = 11.80 Od. Walpha=0.20; WL-0.31; Walpha/WL = 7.33 50 wt% Niarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY