
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please show step by step solution.
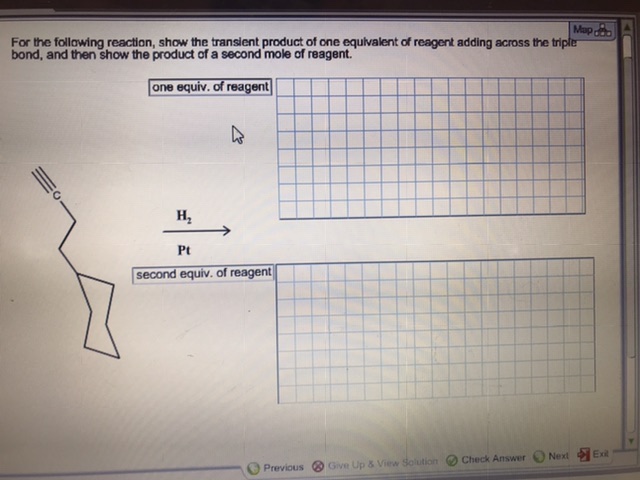
Transcribed Image Text:For the following reaction, show the transient product of one equivalent of reagent adding across the tripler
bond, and then show the product of a second mole of reagent.
one equiv. of reagent
н,
Pt
second equiv. of reagent
Exil
Next
Check Answer
Give Up & View Solution
Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 27% oleum solution is equivalent to a solution that is % SO3 and % H₂SO4arrow_forwardA Solution is What happens if the a b C d in Oz (g) and KNO3 at room temp. is warmed to 75°C Solution Solution 02 bubbles out of Solution bubbles KNO3 precipitates out of solution and gasens out of Sout and KNO3 remain in Solution Saturated in KND3 precipitates out of Solid Gaseous Solid Nothing happensarrow_forwardThe solution is in a clear line on a paperarrow_forward
- The concentration unit one part per billion (one ppb) is equivalent to one _____ of solute per _____ of solution. ng; kg μg; kg μg; g mg; garrow_forwardYou are asked to make 1 liter of a 1:1000 dilution of a solution. How much of the concentrate do you add? How much water?arrow_forwardWrite a step by step procedure to make 500ml of a 0.125 lithium oxide solution. Be sure to include lab equipment you would use.arrow_forward
- How to get 10% HCl solution and 6 M HCl Solution from concentrated HCl solution, which is 12 M or 37% by weight?arrow_forwarddetermine how to prepare 100ml of a 0.250m sodium chloride solution using sodium chloride powerarrow_forwardPrepare 100 mL of a 10mM tris solution using a 1M tris stock (include the amount of water in your answer)arrow_forward
- Concentrated solutions have_ amount of solute lower higher normal abnormalarrow_forwardThe molarity of 25.0 % by mass HCl solution is _______. The density of the HCl solution is 1.20 g/mLarrow_forwardRank the following aqueous solutions from lowest predicted boiling point to highest. In the case of solutions containing aqueous ions, assume there is no ion clustering in the solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY