
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
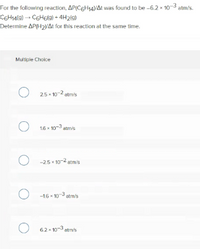
Transcribed Image Text:For the following reaction, AP(C6H14VAt was found to be -6.2 x 10-3 atm/s.
COHA(@) → C6H6(g) + 4H219)
Determine AP(H2NAt for this reaction at the same time.
Multiple Choice
O 25- 10-2 atr's
O 16 - 103 atmis
O -25 10-2 atm/s
O -16 - 103 stm/s
O 62-10-3 atm's
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9.96 Most first aid "cold packs" are based on the endothermic dissolution of ammonium nitrate in water: NH4NO3(s)NH4+(aq)+NO3(aq) H= 25.69 kJ A particular cold pack contains 50.0 g of NH4NO3 and 125.0 g of water. When the pack is squeezed, the NH4NO3dissolves in the water. If the pack and its contents are initially at 24.0°C, what is the lowest temperature that this bag could reach? (Assume that the ammonium nitrate solution has a specific heat of 4.25J g-l K-l, and that the heat capacity of the bag itself is small enough to be neglected.)arrow_forwardMonochloroethane (C2H5Cl) can be produced by the direct reaction of ethane gas (C2H6) with chlorine gas or by the reaction of ethylene gas (C2H4) with hydrogen chloride gas. The second reaction gives almost a 100% yield of pure C2H5Cl at a rapid rate without catalysis. The first method requires light as an energy source or the reaction would not occur. Yet G for the first reaction is considerably more negative than G for the second reaction. Explain how this can be so.arrow_forward5.12. True or false: If all the partial pressures of reactants and products drop by half, the value of Q drops by half. Give an example of a chemical reaction to support your answer.arrow_forward
- The only stress (change) that also changes the value of K is a change in temperature. For an exothermic reaction, how does the equilibrium position change as temperature increases, and what happens to the value of K? Answer the same questions for an endothermic reaction. If the value of K increases with a decrease in temperature, is the reaction exothermic or endothermic? Explain.arrow_forwardCalculate H when a 38-g sample of glucose, C6H12O6(s), burns in excess O2(g) to form CO2(g) and H2O() in a reaction at constant pressure and 298.15 K.arrow_forwardData in the table were collected at 540 K for the following reaction: CO(g) + NO2(g) CO2(g) + NO(g) Using the data in the table: (a) Determine the reaction order with respect to each reactant. (b) Derive the rate equation. (c) Calculate the rate constant, giving the correct units for k.arrow_forward
- Old-fashioned smelling salts consist of ammonium carbonate, (NH4)2CO3. The reaction for the decomposition of ammonium carbonate (NH4)2CO3(s)2NH3(g)+CO(g)+H2O(g) is endothermic. Would the smell of ammonia increase or decrease as the temperature is increased?arrow_forwardThe following equation represents a reversible decomposition: CaCO3(s)CaO(s)+CO2(g) Under what conditions will decomposition in a closed container proceed to completion so that no CaCO3 remains?arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forward
- For the vaporization of one mole of bromine at 59C, determine (a) H (Table 8.2) (b) PV(c) Earrow_forwardAmoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small animals, the pharmacist or veterinarian must suspend it in water, so that it can be administered orally with a medicine dropper. The label says to dispose of unused suspension after 14 days. It also points out that refrigeration is required. In the context of this chapter, what is implied in the latter two statements?arrow_forwardCalculate G for the dissolution of both sodium chloride and silver chloride using data from Appendix E. Explain how the values you obtain relate to the solubility rules for these substances.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning