
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Help
100% 47
Tue 4:
Public Health Ch
HSC 258 - Major Projec X
Mind Tap - Cengage Lea X
C The Illustration To The
d%355750828934189288909969212&elSBN=9781305657571&id=1061392007&nbld=D21...
Tp
Q Search this ce
References
Use the References to access important values if needed for this question.
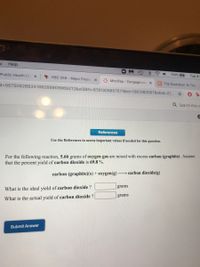
For the following reaction, 5.66 grams of oxygen gas are mixed with excess carbon (graphite) . Assume
that the percent yield of carbon dioxide is 69.8 %.
→ carbon dioxide(g)
carbon (graphite)(s) + oxygen(g)
grams
What is the ideal yield of carbon dioxide ?
grams
What is the actual yield of carbon dioxide ?
Submit Answer
Expert Solution
arrow_forward
Step 1
Given
- mass of Oxygen = 5.66 grams
- percent yield of carbon dioxide = 69.8%
Required data -
- molar mass of Oxygen gas, O2 = 32 g/mol
- molar mass of Carbon dioxide, CO2 = 44 g/mol
To find : a) ideal yield of carbon dioxide in grams
b) actual yield of carbon dioxide in grams
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the reaction of 10.00 mL of FeCl3(aq) and 12.4 g of K2C204(ag), you calculate that 9.58 g of K3FEC2043H2O(s) will be produced. What does 9.58 g represent? percent yield limiting reactant actual yield molar mass theoretical yieldarrow_forwardCH3OH can be synthesized by the reaction : CO (g) + 2H2 (g) -----> CH3OH (g) How many liters of CO gas, measured under the same conditions, are required ?arrow_forwardDraw the Lewis structure created by the curved arrow. Write in formal charges wherever they are not equal to zero H :N=C-C-Br: c_d Harrow_forward
- Given the following three sequential reactions: 1) N2 (g) + 3H2(g) -> 2NH3 (g) 2) 4NH3 (g) + 5O2 (g) -> 4NO(g) + 6H2O (g) 3) 2NO(g) +O2(g) -> 2NO2 (g) What mass of hydrogen gas is needed to produce 108.0 kg of nitrogen dioxide?arrow_forwardWhat mass of CO2 is produced from the combustion of 1 gal of gasoline? The chemical formula of gasoline can be approximated as C8H18. Assume that there are 2801g of gasoline per gallon.arrow_forwardSolid phosphorus P and chlorine Cl2 gas react to form solid phosphorus pentachloride PCl5 . Suppose you have 9.0 mol of P and 1.0 mol of Cl2 in a reactor. Could half the P react? yes no If you answered yes, calculate how many moles of PCl5 would be produced after half the P was used up. Round your answer to the nearest 0.1 mol . molarrow_forward
- Ethylene and chlorine react to form ethylene dichloride, like this: CH₂CH₂(g) + Cl₂(g) → CH₂CH₂Cl₂(g) Use this chemical equation to answer the questions in the table below. Suppose 55.0 mmol of CH₂CH₂ and 55.0 mmol of Cl₂ are added to an empty flask. How much CH₂CH₂ will be in the flask at equilibrium? Suppose 120. mmol of CH₂CH₂Cl2 are added to an empty flask. How much CH₂CH₂ will be in the flask at equilibrium? None. Some, but less than 55.0 mmol. 55.0 mmol. More than 55.0 mmol. None. Some, but less than 120. mmol. O 120. mmol. O More than 120. mmol. 00000arrow_forwardA reaction vessel contains14.1 g of CO and 14.1g of O2. How many grams of CO2 could be produced according to the following unbalanced reaction? CO +0, → CO2 CO+O2arrow_forwardkey carbon hydrogen nitrogen sulfur охудen chlorine Suppose the following chemical reaction can take place in this mixture: CS,(9)+30,(9) → CO2(9)+2 SO2(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula:arrow_forward
- 2NO(g) + O2(g) → 2NO2(g) You are provided with 4.12 molmol of nitrogen monoxide gas. Using the balanced chemical equation completed in Part A, determine how many moles of oxygen gas are needed to completely react with the nitrogen monoxide gas and how many moles of nitrogen dioxide are formed as a result? Ammonia and oxygen react to form nitrogen monoxide and water. Construct your own balanced equation to determine the amount of NO and H2O that would form when 2.80 molmol NH3 and 4.64 mol O2 react. Express the amounts in moles to two decimal places separated by a comma.arrow_forwardBalance the equation below. CO(g) + O2(g) → CO2(g) How many moles of CO(g) are required to react completely with 1 mole of O2(g)? Enter your response in moles (mol) to the nearest 0.1 mol.arrow_forwardFor the following reaction, 3.37 grams of hydrogen gas are mixed with excess ethylene (C₂H4). The reaction yields 44.1 grams of ethane (C₂H6). hydrogen (g) + ethylene (C₂H4) (g). →→→→ethane (C₂H6) (g) What is the theoretical yield of ethane (C₂H6) ? What is the percent yield for this reaction ? Submit Answer % grams Retry Entire Group 2 more group attempts remainingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY