
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
12
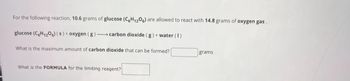
Transcribed Image Text:For the following reaction, 10.6 grams of glucose (C6H1206) are allowed to react with 14.8 grams of oxygen gas.
glucose (C6H12O6) (s) + oxygen (g)-carbon dioxide (g) + water (1)
What is the maximum amount of carbon dioxide that can be formed?
grams
What is the FORMULA for the limiting reagent?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 66 Match the description with the name of the drug. [Sorry there is no partial credit for matching questions) what oplate is found in some cough syrups? What drug is used to treat heroin overdosing? What drug is used to treat heroin addiction, but it is still addictive What drug is used in a patch for chronic pain management? Tolerance for a drug means that over time the dosage can be decreased to have the same effect as before. True False Opiates are all addictive True False Methadone answer not given novocaine "Narcan" or naloxone codeine fentanyl Endorphins and morphine bind to the same receptor sites in the brain True False Acupuncture causes the body to produce endorphins which is the body's own pain killers True False The histamine molecule that sits on the receptor sites in the respiratory system is a different molecule than the one that affects the receptor sites in the stomach True Falsearrow_forward'N Brz NaOH N- ●arrow_forward4. You are trying to determine the volume of your setup according to the procedure in the lab manual. You have determined the following: Mass Standard CaCO3 0.4453 g % CaCO3 Pi Pf Ti Tf 99.6 % 50.5 kPa 129.5 kPa 20.33 °C 21.88 °C What is the volume of the setup in L? The volume is 0.138 Larrow_forward
- 14arrow_forwardWater softening is necessary to prevent clog in plumbing and prevent formation of scum in bathrooms and sinks. O True O Falsearrow_forward3. Which process can separate a mixture of finely powdered iron and wheat flour? A. distillation 3. B. filtration C. using a magnet D. sieving E. sublimationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY