
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please don't provide handwriting solution
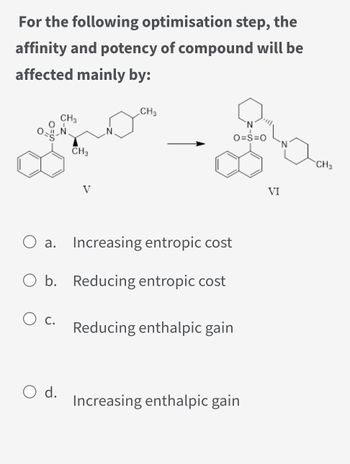
Transcribed Image Text:For the following optimisation step, the
affinity and potency of compound will be
affected mainly by:
0-g-
O c.
CH3
O d.
CH3
O a. Increasing entropic cost
O b. Reducing entropic cost
CH3
Reducing enthalpic gain
O=S=O
Increasing enthalpic gain
VI
CH 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following reaction scheme is expected to atford the tollowing product? HO ОН O The following scheme: 1. Mg, Et,0 Br 2. H H. 3. H;O* O The following scheme: 1. Mg. Et.O HO Br H. H30* O The following scheme: 1. TBS-CI, imid., CH CI, HO Br 2 Mg. Et,0 3. H. 4 H30* 5 Bu N*F THF O The following scheme: 1. Mg. Et,O Br 2. 3. H3O* O The following scheme: 1 BrMg 2 H;0 astimearrow_forwardtext Nu Whe ofect wokl hgh presure have on the proketon of NOg? Why? 4 Wharwoud be the efat of ach of the fulowing on the equilbrium iimvolving the reacion o coke (C (s) when e prokre CO and H C) H,0) cog)+ H,(E) Akten of steam An increase in pvessure Removal of H as it is prodced Akng a catalst S he birding of axygen to hemagbbin (abbreviated Hb). giving oxyhemogldbin (HbO,) s partially glaed by he compk ad tcan be summarized as bolows: contraon of H and CO, in the blood. Although the equilibrum is very HbO, H CO, co,HbH + o, Accordg to Le Chaelers Princpe what wauld be the effect of each of the following on the squban? a The produc on of ac acid (whch contairs Hr) and CO, in a muscle during vigorous exercise? ng besh oxygen aiched a?arrow_forwardNernst equation for pair IO3 |I': RT E = E° + nF In a(10;)a°(H*) a(I¯) RT a(IO;)a(H") E = E° + nF a(I") RT E = E° –- nF In a(IO; )a°(H*) a(I¯) nF a(IO,)a°(H*) lg RT E = E° + a(I¯)arrow_forward
- Given the following reversible reactions and Kc values: (I) Self-ionization of water: H2O + H2O H3O+ + OH-, Kc = Kw = 1.0 x 10-14. (II) Ionization of a weak base: NH3 + H2O NH4+ + OH-, Kc (= Kb) = 1.8 x 10-5. a) Determine the Kc for the reaction: NH3 + H3O+ NH4+ + H2O b) Would you assess the Kc value as LARGE or small? Does your assessment make sense for this reaction, i.e. think about the species involved?arrow_forwardMultiple choice question Help?arrow_forwardConsider the following reactions: A 2B 2D AH2 E C + D AH3 AH for the reaction A 20 + E is: Select one: a. ΔΗ +2ΔΗ, +ΔΗ, b. ΔΗ +2 ΔΗ, -2 ΔΗ3 c. ΔΗ +2 ΔΗ, ΔΗ3 d. ΔΗ +2 ΔΗ. e. ΔΗ-ΔΗ, +2 ΔΗ,arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
