
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
12. No AI Use
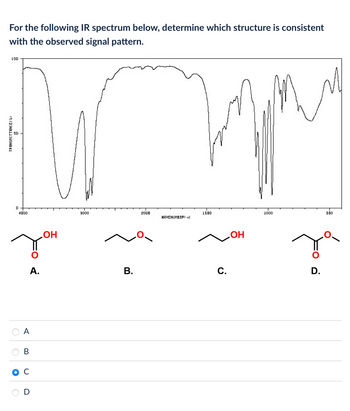
Transcribed Image Text:For the following IR spectrum below, determine which structure is consistent
with the observed signal pattern.
TRANSMITTANCEIX
LOD
D
4000
A
B
A.
OH
3000
2000
1500
HAVENUMBERI-II
B.
C.
OH
1000
500
D.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- What structure would match the data ?arrow_forwardA student calibrated the mini-spectrophotometer with a blank solution, and then went to check their solution. They got a fingerprint on the cuvette while filling it. Because of this, the expected reading for the absorbance would be too high which would cause the calculated value of K, to be too higharrow_forwardWhich structure does this IR belong to and why?arrow_forward
- Annotate IR spectrum and determine structure :)arrow_forwardAnnotate IR spectrum and determine structure :)arrow_forwardIdentify which functional groups are suggested by the signals indicated by each of the arrows. Include whether the signal is due to a stretching vibration or a bending vibration.arrow_forward
- Any information on these spectrums would be helpful! Trying to figure out what specific type of chemical each spectrum belongs to.arrow_forwardAnswer the question concisely. Maximum of 5 sentences. Differentiate spectroscopy and spectrophotometry. Cite an example and discuss it in a chemical perspective.arrow_forwardIdentify the molecules and why you think it is the molecule.arrow_forward
- • Deduce a structure consistent with the IR spectrum. There may be more than one answer. MCRONS 200 2400 1600 s00 WAVEMM 4000 2000 00 200 1000 STRANSMITTANCEarrow_forwardJM 4: C₂H₁2O 100 % Transmittance 95 90 85 80 75 70 65 60 Janne DRAW ONE POSSIBLE STRUCTURE ATR 4000 3750 Sigma-A ALL RIGHTS RESERVED 3500 any 1000 1250 1500 1750 2000 2250 2500 Wavenumbers (cm-1) © 2023, Sigma-Aldrich Co. ALL RIGHTS RESERVED 2750 3000 3250 750 500arrow_forwardPlease don't provide handwriting solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning