
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:O voltage
from left to right (----->)
.39 V; electrons move
O voltage
from left to right (----->)
.41 V; electrons move
O voltage
from left to right (----->)
1.99 V; electrons move
O voltage
from right to left (<-----)
1.99 V; electrons move
%D
O voltage
from right to left (<-----)
.39 V; electrons move
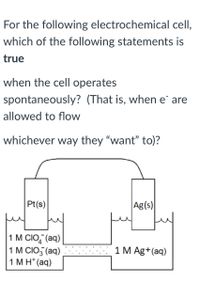
Transcribed Image Text:For the following electrochemical cell,
which of the following statements is
true
when the cell operates
spontaneously? (That is, when e are
allowed to flow
whichever way they "want" to)?
Pt(s)
Ag(s)
1 M CIO,"(aq)
1 M CIO, (aq)
1 M H* (aq)
1 M Ag+(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A voltaic electrochemical cell is described below. Identify the anode and cathode, label the diagram below (or sketch your own), write the overall balanced reaction, and calculate E°cell. Sn(s) | Sn2+(aq) || NO(g) | NO3−(aq), H+(aq) | Pt(s)arrow_forwardWrite the net cell equation for the electrochemical cell. Phases are optional. Do not include the concentrations. Co(s)|Co #(aq, 0.0155 M) || Ag*(aq, 1.50 M)|Ag(s) net cell equation:arrow_forwardA galvanic cell is powered by the following redox reaction: 2 Br,(1) + N,H,(aq) + 4OH (aq) 4 Br (aq) + N,(g) + 4 H,O(1) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. ローロ e Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E - Round your answer to 2 decimal places. のarrow_forward
- Write the half-reactions as they occur at each electrode and the net cell reaction for this electrochemical cell containing indium and cadmium. In(s)||In3+(aq)‖‖Cd2+(aq)||Cd(s)In(s)|In3+(aq)‖Cd2+(aq)|Cd(s) anode: cathode: net cell reaction:arrow_forwardA voltaic cell employs the following redox reaction: 2 Fe3+ Calculate the cell potential at 25 °C under each of the following conditions. (ag) + 3 Mg (s) → 2 Fe (s) + 3 Mg²+ (aq) Part C [Fe+] = 3.10 M ; [Mg²+] = 1.2x10-3 M Ecell Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remainingarrow_forwardWhat is e cell of the following electrochemical cell ag(s) / agcl (s), KCl (aq, 1 M) // H2 (g, 1 atm), pH 7.00 aq / Pt (s)arrow_forward
- A voltaic cell is constructed in which the following cell reaction occurs. The half-cell compartments are connected by a salt bridge. Pb2+(aq) + Mn(s) Pb(s) + Mn2+(aq) The anode reaction is: The cathode reaction is: + + In the external circuit, electrons migrate e the Mn|Mn2+ electrode e the Pb|Pb2+ electrode. In the salt bridge, anions migrate e the Pb|Pb²+ compartment e the Mn|Mn2+ compartment. +arrow_forwardWrite the cell notation for an electrochemical cell consisting of an anode where Mg (s) is oxidized to Mg²+ (aq) and a cathode where Sn²+ (aq) is reduced to Sn (s). Assume all aqueous solutions have a concentration of 1 mol/L.arrow_forwardUse the tabulated half-cell potentials to calculate ΔG° for the following reaction. (Hint: Before you being, you must determine whether or not the reaction is balanced.)I2(s) + Fe(s) → Fe3+(aq) + I⁻(aq) (a)‒1.1×102kJ (b) +4.9×101kJ (c) ‒9.7×101kJ (d) ‒3.3×102kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY