
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I believe that this rxn will proceed via Eliminatin but I can't draw the mechanism.
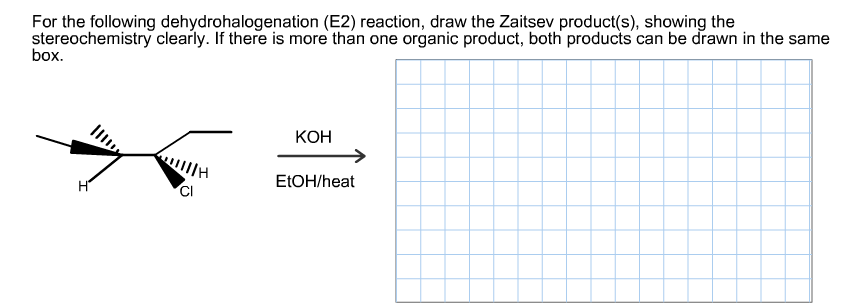
Transcribed Image Text:For the following dehydrohalogenation (E2) reaction, draw the Zaitsev product(s), showing the
stereochemistry clearly. If there is more than one organic product, both products can be drawn in the same
box
КОН
ll
E1OH/heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8) Consider the below desired transformation. Develop a retrosynthesis and forward synthesis that would successfully accomplish it. doarrow_forwardThe use of sodium iodide in acetone promotes SN2 mechanism for alkyl halide resulting in the formation of insoluble salts in the mixture. True Falsearrow_forwardOH H* 요 ey" (Hint: reverse cyclic aldol) Harrow_forward
- Construct a mechanism for the following reaction.arrow_forwardDraw the mechanism and organic product(s) formed when CH3CH2CH2OH is treated with HCl + ZnCl2.arrow_forwardications to the substrate. These pathways be regulated so that the small molecules are ent in appropriate amounts. Part A OPO Which of the following statements is most likely to be true in the case of the feedback-regulated enzymatic pathway shown? P1 • View Available Hint(s) E2 P2 O PO binds E3 and activates it. E3 P3 O P2 binds E2 and activates it. P3 binds E1 and deactivates it. O P2 binds E1 and activates it. O P3 binds E2 and deactivates it. Submitarrow_forward
- Kk.319.arrow_forwardhow do i draw the complete SN1 mechanism (with curved arrows) of the reaction of 2-bromobutane withethanol.arrow_forward1) Which substrate would react most rapidly in an SN2 reaction? O CH3CH2CH2CH=CHBr OCH2=CHCH2CH2CH2Br CH3CH2CH=CHCH2Br BrCH2CH2CH=CHCH3 OCH3CHBRCH=CHCH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY