
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
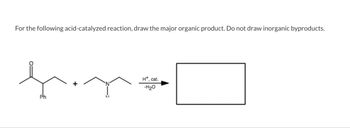
Transcribed Image Text:For the following acid-catalyzed reaction, draw the major organic product. Do not draw inorganic byproducts.
find
Ph
H+, cat.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. In this reaction, a pi bond acts as a Bronsted base. Using the provided starting structures, draw the curved electron- pushing arrows for the acid-base reaction. Be sure to account for all bond-breaking and bond-making steps. Then draw the organic product of the reaction. + Include all lone pairs in the structures. Ignore inorganic byproducts and counterions. I I HH :CI. co THE I Select to Edit Arrowsarrow_forward5 pleasearrow_forwardComplete the following malonic (di)ester synthesis by showing all reagents and reaction conditions required for the transformation. Balance the reaction. H2 H3C. CH H3CO OCH3 CH3 :o:arrow_forward
- From the table of available reagents select the one(s) you would use to convert butanoic acid to each of the following products: (Use the minimum number of steps; from one to six are required. List reagents by letter in the order that they are used; example: fa. NOTE: Assume that a normal aqueous (or mild acid) workup follows each reaction; you do not need to add reagents for the workup.) a. 1-butene b. N-methylpentanamidearrow_forwardDraw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. I :O: NaOH :O: Select to Draw Harrow_forwardWhy are the carboxylic acid groups added in a syn orientation during hydrolysis for the following reaction?arrow_forward
- K Draw the initial organic product of the Bronsted acid- base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. H3O+ Drawing Question 5 Atoms,arrow_forwarddraw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions.arrow_forwardFrom the table of available reagents select the one(s) you would use to convert butanoic acid to each of the following products: (Use the minimum number of steps; from one to six are required. List reagents by letter in the order that they are used; example: fa. NOTE: Assume that a normal aqueous (or mild acid) workup follows each reaction; you do not need to add reagents for the workup.)arrow_forward
- Draw the structure of the neutral nitrogen containing organic product formed in the following reaction. Do not include counterions or byproducts. -> NH2 (2 equivs.)arrow_forwardFrom the table of available reagents select the one(s) you would use to convert butanoic acid to each of the following products: (Use the minimum number of steps; from one to six are required. List reagents by letter in the order that they are used; example: fa. NOTE: Assume that a normal aqueous (or mild acid) workup follows each reaction; you do not need to add reagents for the workup.) Reagents Available a. benzene/AlCl3 d. H₂O, H₂SO4 b. (CH3)2CuLi e. H₂, Pd/C c. CH3NH₂ f. K* -OC(CH3)3 1-bromobutane butanenitrile g. LiAlH4 h. NaCN i. NH3 j. PBr3 k. Dess-Martin periodinane I. SOCI₂arrow_forwardProvide a mechanism that accounts for the outcome of the following hydrolysis reaction. H3O+ ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY