
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please confirm if my answer is correct or not. Due to the need for this to be correct I would like you to provide me with a confidence score in you answer on a scale from 1 to 10 how confident are you in your answer. Please be sure to double check your answer
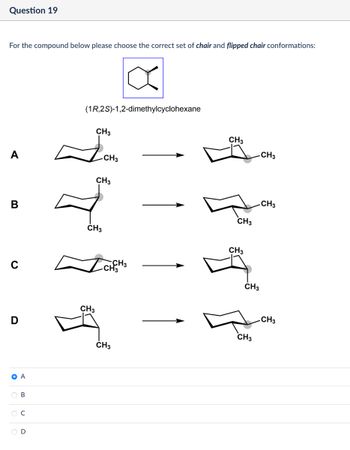
Transcribed Image Text:Question 19
For the compound below please choose the correct set of chair and flipped chair conformations:
(1R,2S)-1,2-dimethylcyclohexane
CH3
A
CH3
CH3
B
CH3
CH3
-CH3
CH3
CH3
CH3
с
-CH3
D
• A
B
ი
C
D
CH3
CH3
CH3
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- there can only be one answer per question for 4 and 5 you put 2 answers per eacharrow_forwardUsing a 25mL pipette, you prepare a standard solution by pipetting 25.00mL of the standard iron solution[0.25g/L] into a 500mL volumetric flask. Then, using a 10mL pipette you transfer a 4mL aliquot of this solution into a 50mL volumetric flask and dilute up to the mark. Calculate the % uncertainty. Question 1 options: 0.65 0.75 0.0075 0.45arrow_forwardSuppose that in a random selection of 100 colored candies, 25% of them are blue. The candy company claims that thepercentage of blue candies is equal to 24%. Use a 0.05 significance level to test that claim.Identify the null and alternative hypotheses for this test. Choose the correct answer below.arrow_forward
- A sample was placed on a chromatography column. Methylene chloride was used as the eluting solvent. No separation of the components in the sample was observed. What must have been happening during this experiment? How would you change the experiment to overcome this problem?arrow_forwardPlease answer.arrow_forwardPlease name the following compoundsarrow_forward
- Give detailed Solution with explanation needed (don't give Handwritten answerarrow_forwardPlease let me know which 2 answers are correct, an explanation including evidencearrow_forwardWhen the math expression below is simplified, how many sig figs should the result have? 91.69-90.98 6.35 + 4.44 4 3 O 2 0 1arrow_forward
- See the following table of concentration, green intensity, blank. Based on these data, convert intensity data into absorbance. Make a plot of absorbance versus relative concentration. Find the equation for the best fit line on the plot. Concentration (M) Green intensity (I) Blank (lo) Absorbance (-log 1/10) 0.000 234 234 0.100 228 234 0.200 221 234 0.300 216 234 0.400 211 234 0.500 203 234 0.600 199 234 0.700 191 234 0.800 185 234 0.900 179 234 1.000 174 234 a) Show the best fit line as the form of y = ax + b for your answer. You do not need to show your plot. How much is the slope (m) in this best fit line as the form of y = mx + b in your answer? Type your answer...arrow_forwardWhich of the following would be a correct interpretation of a 99% confidence interval such as 4.1arrow_forwardA student determines the concentration of an unknown sample using two different methods. Each method has three different trials; the average, standard deviation, and percent error produced by each method is listed below. Method 1 Average: 10.56 M NaOH Standard deviation: 1.35 M NaOH Percent error: 1.25% Method 2 Average: 10.92 M NaOH Standard deviation: 1.02 M NaOH Percent error: 3.45% Which method was more accurate? How can you tell?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY