
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
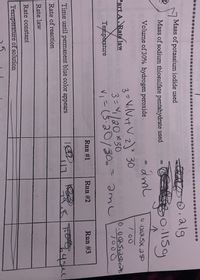
For runs #1-#3 , calculate the rates of reaction. The
rate= 1/2 x molarity of sodium thiosulfate pentahydrate/ reaction time in seconds
Indicate the units for each reaction rate.
I know the the times are a little hard to read so here they are : run #1:117 seconds , run #2: 79.8 seconds ,run #3 :64 seconds.

Transcribed Image Text:k********************
关
**************
*****
*********
Mass of potassium iodide used
0.alg
0:115g
Mass of sodium thiosulfate pentahydrate used
%3D
Volume of 30% hydrogen peroxide
0.0025x 2so
3=V,(Vit Vz)* 30
3=V/20*30
vi=(3.20/30=
100
Part A.Rate law
0.00ASx250xas
1000
amu
%3D
Temperature
%3D
Run #1
Run #2
Run #3
Time until permanent blue color appears
200
Rate of reaction
Rate law
Rate constant
Temperature of solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some measurements of the initial rate of a certain reaction are given in the table below. Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardConsider the following reaction: 4 NH_{3} + 5 O_{2} → 4 NO + 6 H_{2}O The rate of consumption of NH_3 is -1.4×10^{-5} M/s. Calculate the relative reaction rate. Use “E” for scientific notation. Do not enter units as part of your answer.arrow_forwardFor the reaction A → products, concentration and time data were collected. Enter these data into the graphing tool to determine the reaction order. t Graphing Tool 0.0 25.0 50.0 73.0 [A] 3.40 2.28 1.53 1.02 In[A] 1.223775 0.824175 0.425268 0.019803 Clear All Data 1/[A] 0.294118 0.438596 0.653595 0.980392 [A] 3.4 2.822 2.244 1.666 1.088 0.51 [A] vs. t to 0 14.6 t (s) 0.0 25.0 50.0 75.0 In[A] vs. t 29.2 t 43.8 1/[A] vs. t 58.4 [A] (M) 3.40 2.28 1.53 1.02 73arrow_forward
- 2C1₂0, (g) → 2Cl₂ (g) +50₂ (g) He fills a reaction vessel with C1₂O5 and measures its concentration as the reaction proceeds: time (seconds) 0 0.10 0.20 0.30 [C₁₂05] 0.0500M 0.0322M 0.0207 M 0.0133M 0.40 0.00858M Use this data to answer the following questions. Write the rate law for this reaction. Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. rate = k [C₁₂0₂] k = 0 x10 □・□ Xarrow_forwardA chemistry graduate student is studying the rate of this reaction: She fills a reaction vessel with and measures its concentration as the reaction proceeds: time (seconds) Use this data to answer the following questions. Write the rate law for this reaction. rate Calculate the value of the rate constant . Round your answer to significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardCan you help me correcting this questions in the picture with the notes below please? Notes: In question 1a, the rate law is fine but should have partial pressures, not concentrations. In 1b, the units of the answer are incorrect; they should have "atm" in it. Hint: in the denominator of the calculation is atm^3.arrow_forward
- Write the balanced reaction that corresponds to the data in graph.arrow_forward22. Based on the reaction rate data shown in Table 1, plot a properly labelled graph of Concentration vs. Time in the space provided. Draw a curve to connect the points. Table 1: Reaction rate Time (s) 0 1 2 3 4 5 6 7 8 9 10 Concentration (mol/L) 0 0.15 0.20 0.30 0.50 0.80 0.90 0.95 0.98 0.99 1.00 This curve should ascend over time. Conc vs 0.10 6.9 0.8 0.7 0.6 G.S 6.4 0.3 0.2 O. I • time 10 (mol/L) Concentration 4. Timeros 8 12 23. a) Based on the reaction rate data in Table 1, determine the average rate of chemical production between 2 s and 7 s. b) On the above graph, draw a line segment to represent the details in this question. Note: Show all calculations, follow the rules of significant figures, and box your final answer.arrow_forwardGeneral Chemistry 4th Edition McQuarrie Rock Gallogly University Science Books presented by Macmillan Learning Initial-rate data at a certain temperature is given in the table for the reaction N,0,(g) → NO(g) + NO,(g) [N,03lo(M) Initial rate (M/s) 0.100 0.720 0.200 1.440 0.300 2.160 Determine the value and units of the rate constant. units: k = Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry | Publisher: University Scien MacBook Proarrow_forward
- The following reaction occurs in your car's exhaust system catalytic converter:Heat + 2CO + O2 + Pt → 2CO2 + PtWhat is the catalyst?arrow_forwardPlease provide explarrow_forwardHere is a graph of the molarity of bromine Br2 in a reaction vessel during a certain chemical reaction. If Br2 is being created or destroyed, what is the rate at which it is being created or destroyed 10 seconds after the reaction starts? Round your answer to 2 significant digits. If Br2 is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 10 seconds of the reaction? Round to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY