
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
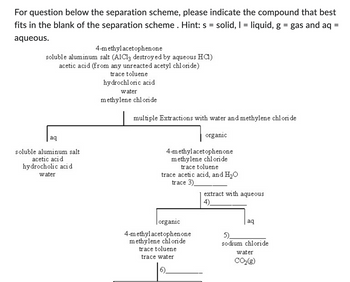
Transcribed Image Text:For question below the separation scheme, please indicate the compound that best
fits in the blank of the separation scheme . Hint: s = solid, 1 = liquid, g = gas and aq =
aqueous.
4-methylacetophenone
soluble aluminum salt (A1C13 destroyed by aqueous HC1)
acetic acid (from any unreacted acetyl chloride)
trace toluene
hydrochloric acid
water
methylene chloride
aq
soluble aluminum salt
acetic acid.
hydrocholic acid
water
multiple Extractions with water and methylene chloride
organic
4-methylacetophenone
methylene chloride
trace toluene
trace acetic acid, and H₂O
trace 3)
organic
4-methylacetophenone
methylene chloride
trace toluene
trace water
extract with aqueous
4)
aq
5)
sodium chloride
water
CO₂(g)
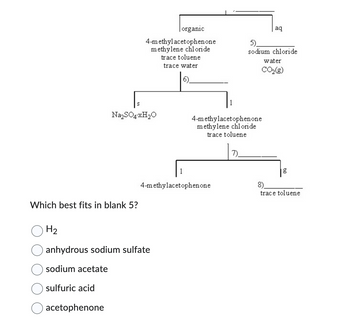
Transcribed Image Text:Which best fits in blank 5?
H₂
organic
4-methylacetophenone
methylene chloride
trace toluene
trace water
Na₂SO4-xH₂O
acetophenone
anhydrous sodium sulfate
sodium acetate
sulfuric acid
4-methylacetophenone
4-methylacetophenone
methylene chloride
trace toluene
7).
aq
5)
sodium chloride
water
CO₂(g)
g
8)
trace toluene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Classify these solvents as either protic or aprotic solvents.arrow_forwardStarting materials Br Reagents OH 1 H3C OH 2 Br 6 Br 7 OH OH TOH OH 8 5 H a Mg / dry ether e Aqueous H2SO4 at reflux i KMnO4 / H3O+ b 1. CO2 2. acidic workup of Conc. HCI or HCI (gas) j Na2CrO4 / aqueous H2SO4 C NaCN/THF or DMF g PBr3 K 1. BH3/THF 2. H₂O2/aq. NaOH d NaCN/dil. aqueous H2SO4 h KOH alcohol |arrow_forwardThe following flow chart outlines an acid/base extraction of two compounds. Identify which compound corresponds to each letter G - L. Match the appropriate structure number to the corresponding letter box. Possible answers 1- 7 are given in the bank below the flow chart; some maybe used more than once and not all may be used.arrow_forward
- Acid 3- Fluro benzoic NO2 NO2 Nitro ben zene 1,3- Dinitro benzene HO. Fina! (3- Fluoro kenzayamine) NH2 3- Fluoro ben zamidearrow_forwardI want you to solve for HAc using the ammonia sample as reference.arrow_forward4. Below is an acid-base extraction flow chart. The four compounds seen in the first box was mixed and dissolved in dichloromethane. Complete the flow chart by drawing (bond-line structure) the organic compound that is found in each layer upon each addition. Draw in any relevant charges. OH CN 10% HCI ORGANIC AQUEOUS 10% NaHCO3 AQUEOUS ORGANIC 10% КОН AQUEOUS ORGANICarrow_forward
- identify the unknowns as 1°ROH, 2°ROH, 3°ROH, ether or phenol. Solubility in Solubility in Вayer's Chromic acid Ferric chloride Lucas test H20 NaOH oxidation oxidation test Visible + result SAMPLES Ethanol ++ ++ ++ 2-Butanol + ++ ++ tert-Butanol + ++ Phenol ++ Diethyl ether Unknown 1 + ++ ++ Unknown 2 Unknown 3 + ++ Legend: ++ → fast reaction/completely soluble; + > slow reaction/slightly soluble; - → no reaction/insoluble Identities of Unknown Samples Unknown 1: Unknown 2: Unknown 3: Write Pertinent chemical equation for the following Bayer's oxidation for ethanol: Chromic acid oxidation 2-butanol: Lucas test for tert-Butanol Ferric chloride test for phenolarrow_forward5. Each mixture below contains 1 mL solvent 1, 1 mL of solvent 2, and 40 mg of the solid. For each, indicate the species contained in each layer using the sketched test tube. Solvent 1 CH,CH,-O-CH,CH, H₂O Solvent 2 CH,CH,-O-CH,CH, H₂O CH₂Cl₂ CH₂Cl₂ H₂O IM HCI (aq) H₂C-N Solid NaCl CH3 H₂C HQ NH₂ Test tube Darrow_forward1. Complete the following flowchart. H₂N Aqueous phase Add NaOH pellets OH OH + 1) Dissolved in ether 2) Extracted w/ 3M HCI Aqueous phase Add conc. HCI Organic phase Extracted w/ 3M NaOH Organic phasearrow_forward
- Select all that apply. If dichloromethane (CH2CI2), benzylamine, benzoic acid, and excess aqueous NaOH are mixed in a separatory funnel, what constituent(s) will primarily be in the aqueous phase? Select all that apply.©GML 2020 1. None of these O 2. Sodium benzoate 3. Benzoic acid 4. Benzylamine 5. CH2CI2 O 6. Sodium benzylamine O 7. Sodium hydoxidearrow_forwardI want to explain what happened in this reactionarrow_forwardQuestions 1 and 2 Solvents used are hexane,toluene,acetone,ethanol, and waterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY