
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
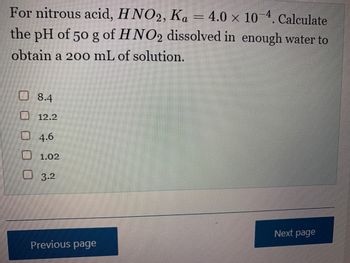
Transcribed Image Text:For nitrous acid, HNO2, Ka 4.0 x 10-4. Calculate
the pH of 50 g of HNO2 dissolved in enough water to
obtain a 200 mL of solution.
8.4
12.2
4.6
1.02
3.2
Previous page
__
Next page
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pOH of [H*]= 2.5 x 10-5 M. none 4.0 x 10-10 9.40 O 4.60 14.0arrow_forward៣ AA Learning E4 CH 16 17 and 18 prod03-cnow-owl.cengagenow.com Learning Learning Learning × Online tea... y dr. marlow... ember L... with We [References] Question 1 1 pt Question 2 2 pts Question 3 1 pt Question 4 2 pts Calculate the pH of a solution that has an ammonium chloride concentration of 0.046 M and an ammonia concentration of 0.045 M. K 1.8 x 10-5 Question 5 1 pt pH = Question 6 2 pts Question 7 1 pt Submit Answer Try Another Version 2 item attempts remaining Question 8 2 pts Question 9 1 ptarrow_forwardThe Ka for a weak acid is 1.2*10^-8. a. What is the equilibrium concentration of OH- in a 0.0020 M solution of the conjugate base? b. What is the equilibrium concentration of the undissociated conjugate base in a 0.0020 M solution of the conjugate base? c. What is the equilibrium pH of a 0.0020 M solution of the conjugate base?arrow_forward
- A sample of water from a stream has [H3O*]=3.1 × 10-9 M. Which statement below accurately describes this water sample? A. The water sample is acidic. B. The water sample is a basic solution. C. The pOH of the water sample is 8.50. D. pH of the water sample is much les than 7.0. E. The pH of the water sample is 7.5.arrow_forwardA. What is the pH of an aqueous solution with a hydrogen ion concentration of [H*] = 8.1 x 10-5 M? pH B. What is the hydroxide ion concentration, [OH], in an aqueous solution with a hydrogen ion concentration of [H*] = 8.1 x 10-5 M? [OH-] = C. A monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H*(aq) + A-(aq) The equilibrium concentrations of the reactants and products are [HA] = 0.190 M, [H*] = 4.00 x 104 M, and [A-] = 4.00 x 104 M. Calculate the Ką value for the acid HA. Ka = TOOLS x10"arrow_forwardHydrochloric acid is a strong acid. In a 0.01M solution, what are the pH value and the concentration of hydroxide ion? Select one: a. pH = 0.01 [OH-] = 0.01 mol dm-3 b. pH = 2 [OH-] = 10-12 mol dm-3 c. pH = 2 [OH-] = 12 mol dm-3 d. pH = 0.01 [OH-] = 12 mol dm-3arrow_forward
- 1. Calculate the pH of a soultion that has a hydrogen ion concentration of 1.25 x 10-4 2. Calculate the [H+] concentration of a soultion with a pH of 11.5 3. Write an equation to illustrate the reaction between nitric acid (HNO3) and barium hydroxide (Ba(OH)2)arrow_forwardPlease help solvearrow_forwardQUESTION 6 Which answer lists the chlorine containing acids in order of decreasing strength, strongest first? A. HCI > HCIO4 > HCIO3 > HCIO2 > HCIO B.HCIO4 > HCIO3 > HCIO2 > HCIO > HCI C. HCIO > HCIO2 > HCIO3 > HCIO4 = HCI D. HCI = HCIO4 > HCIO3 > HCIO2 > HCIO E.HCI > HCIO > HCIO2 > HCIO3 > HCIO4arrow_forward
- Determine the pH of the following solutions: 1. [H+1] = 2.3 X 10-13 M 2. [H+1] = 7.3 X 10-3 M From the answers you obtain, answer the following questions about each solution: 1. Determine whether each solution is an acid or base. Explain your answer. 2. Determine the potential products of the reaction of the solution identified as acid from above and metallic zinc. 3. Determine what color litmus paper will turn for each solution. 4. Determine whether each solution is a (strong acid or base) or a (weak acid or base).arrow_forwardCalculate the pH of each of these solutions. is the solution acidic, basic, or neutral ?. a. a solution with a OH- concentration of 5.5 x 10-12 M b. a solution with a H3O+ concentration of 3.2 x 10-8 M c. a solution with a pOH of 7.00arrow_forwardDetermine the pH of a 7.98 x 10 M Ca(OH)2 solution. Your answer should contain 3 decimal places as this corresponds to 3 significant figures when dealing with logs. PH3Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY