
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
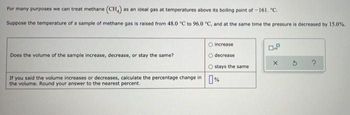
Transcribed Image Text:For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of -161. "C.
Suppose the temperature of a sample of methane gas is raised from 48.0 °C to 96.0 °C, and at the same time the pressure is decreased by 15.0%.
O increase
0.8
Does the volume of the sample increase, decrease, or stay the same?
decrease
X
?
Ostays the same
If you said the volume increases or decreases, calculate the percentage change in %
the volume. Round your answer to the nearest percent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 27.1 g sample of solid CO2 (dry ice) is added to a container at a temperature of 100 K with a volume of 4.2 L.arrow_forward59. A sample of gas isolated from unrefined petroleum contains 90.0% CH4, 8.9% C2H6, and 1.1% C3H8 at a total pressure of 307.2 kPa. What is the partial pressure of each component of this gas? (The percentages given indicate the percent of the total pressure that is due to each component.)arrow_forwardFor many purposes we can treat nitrogen (N2) as an ideal gas at temperatures above its boiling point of -196.C. Suppose the temperature of a sample of nitrogen gas is raised from -88.0C to -79.0C, and at the same time the pressure is decreased by 10%. Does the volume of the sample increase, decrease or stay the same? Calculate the percentage change in the volume. Round your answer to the nearest percent?arrow_forward
- e to search For many purposes we can treat propane (C3H₂) as an ideal gas at temperatures above its boiling point of -42. °C. Suppose the temperature of a sample of propane gas is lowered from 24.0 °C to -18.0 °C, and at the same time the pressure is changed. If the initial pressure was 7.1 atm and the volume increased by 45.0%, what is the final pressure? Round your answer to the correct number of significant digits. atm Continue Eve Fase RK-burial- Sent Papers WE- burial-goateced HE Torner Batavia Justin Sawith x10 X The Grand in Buffalo 1205 Delaware Ave Buffalo NY 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Christopher Mitchell Submit Assignment setup appt for Lole Sucencias Citataarrow_forwardP 8.98 atm Vị 8.95 L T; 610. K P; 4.38 atm V; 6.98 L T; ?K Tf = Karrow_forwardPlease answer this question asap I will rate you for sure.arrow_forward
- For many purposes we can treat butane (C,H10) as an ideal gas at temperatures above its boiling point of – 1. °C. 3 Suppose the pressure on a 8.0 m° sample of butane gas at 11.0°C is reduced to one-third its initial value. O yes Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? olo O no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardFor many purposes we can treat propane (C,H,) a as an ideal gas at temperatures above its boiling point of -42. "C. Suppose the temperature of a sample of propane gas is raised from 90.0 °C to 144.0 °C, and at the same time the pressure is increased by 10.0%. Does the volume of the sample increase, decrease, or stay the same? O increase O decrease O stays the same If you said the volume increases or decreases, calculate the percentage change in % the Round your answer to the percent Xarrow_forwardFor many purposes we can treat methane (CH,) as an ideal gas at temperatures above Its bolling point of-161. °C. Suppose the temperature of a sample of methane gas is ralsed from -21.0 °C to 13.0 °C, and at the same time the pressure is changed. If the Initlal pressure was 2.8 atm and the volume Increased by 30.0%, what is the final pressure? Round your answer to the correct number of significant digits. atm x10arrow_forward
- For many purposes we can treat nitrogen (N,) as an ideal gas at temperatures above its boiling point of -196. °C. Suppose the temperature of a sample of nitrogen gas is lowered from 95.0 °C to 58.0 °C, and at the same time the pressure is increased by 5.0%. O increase Does the volume of the sample increase, decrease, or stay the same? O decrease O stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent. % Explanation Check S26 PM P Search for anything 号 12/6/2020 delata %23 3. & backspece 12 5 71 home 8 E Y U tab ULL K enter 4. caps lock pause nits 回 国国arrow_forwardA sample of methane gas at a pressure of 0.947 atm and a temperature of 29.0 °C, occupies a volume of 711 mL. If the gas is allowed to expand at constant temperature until its pressure is 0.504 atm, the volume of the gas sample will be | mL.arrow_forwardor many purposes we on treat propane (C₂¹) as an ideal gas at temperatures above its boiling point of -42. °C. Suppose the temperature of a sample of propane gas is lowered from 12.0 °C to -31.0 °C, and at the same time the pressure is increased by 5.0%. Does the volume of the sample increase, decrease, or stay the same? O increase O decrease O stays the same If you said the volume increases or decreases, calculate the percentage change in % the volume. Round your answer to the nearest percent. 0 x10 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY