
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ses/228/assignments/389/tasks/97
aps
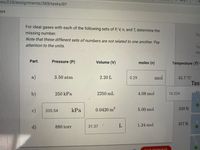
For ideal gases with each of the following sets of P, V, n, and T, determine the
missing number.
Note that these different sets of numbers are not related to one another. Pay
Du
attention to the units.
To
Ma
Part
Pressure (P)
Volume (V)
moles (n)
Temperature (Tra
a)
50atm
2.20 L
0.29
mol
45.7°C
Tasi
b)
250 kPa
2250 mL
4.08 mol
16.224
c)
kPa
0.0420 m3
5.00 mol
339 K
335.54
1.24 mol
357 K
d)
880 torr
31.37
OAVE DESRONSE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the apparatus shown below with the valve initially closed. The volume and pressure in each bulb is indicated on the diagram. The temperature remains constant. N2 H2 Valve 3.00 L 6.00 L 1.00 atm 2.00 atm When the valve is opened the two gases mix uniformly. At that point, what is the partial pressure of hydrogen in the apparatus? O 2.00 atm O 1.00 atm O 1.50 atm O 1.33 atmarrow_forwardFor many purposes we can treat nitrogen (N₂) as an ideal gas at temperatures above its boiling point of -196. °℃. Suppose the temperature of a sample of nitrogen gas is raised from 27.0 °C to 57.0 °C, and at the same time the pressure is increased by 5.0%. Does the volume of the sample increase, decrease, or stay the same? increase decrease stays the same If you said the volume increases or decreases, calculate the percentage change in % the volume. Round your answer to the nearest percent. x10 X Śarrow_forwardA sample of gas isolated from unrefined petroleum contains 90.0% CH4, 8.9% C2H6, and 1.1% C3H8 at a total pressure of 307.2 kPa. What is the partial pressure of each component of this gas? (The percentages given indicatethe percent of the total pressure that is due to each component.)arrow_forward
- For many purposes we can treat ammonia (NH, ) as an ideal gas at temperatures above its boiling point of - 33. °C. Suppose the temperature of a sample of ammonia gas is raised from 50.0 °C to 82.0 °C, and at the same time the pressure is increased by 5.0%. increase Does the volume of the sample increase, decrease, or stay the same? decrease ? stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent. %arrow_forwardA student recovered0.398g of THF vapor in a 125 mL flask. Assuming the vapor is an ideal gas with a molar volume of 22.4 L/mol, calculate the molar mass of THF.arrow_forwardNitesharrow_forward
- 1 atm = 760 torr = 760 mm Hg= 101,325 Pa = 14.69 psi R= 0.0821 L*atm/mol*K 1. List the 5 gases that make up the air we breathe and label which one is in the highest percentage and which is in the second highest percentage Ates of iurenolcuarrow_forwardPlease don't provide handwrittin solution...arrow_forwardFour samples of the same unknown gas G are listed in the table below. Rank these samples in increasing order of ideality. That is, select 1 next to the sample of G that will behave least like an ideal gas, and select 4 next to the sample of G that will behave most like an ideal gas. ? How ideal the sample is: pressure volume temperature sample (atm) (L) (°C) A 25.0 5.0 0.0 (Choose one) В 45.0 5.0 0.0 (Choose one) C 45.0 5.0 - 10.0 (Choose one) 35.0 3.0 0.0 (Choose one) ▼arrow_forward
- 59. A sample of gas isolated from unrefined petroleum contains 90.0% CH4, 8.9% C2H6, and 1.1% C3H8 at a total pressure of 307.2 kPa. What is the partial pressure of each component of this gas? (The percentages given indicate the percent of the total pressure that is due to each component.)arrow_forward19.arrow_forwardA gas mixture containing only O2 and N2. If the mole fraction of O2 is 0.37, and the total pressure is 2.4 atm, what is the partial pressure of N2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY