
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
For H2(g)+Br2(g)↽−−⇀2HBr(g),H2(g)+Br2(g)↽−−⇀2HBr(g), ?=7.2×10−4K=7.2×10−4 at 1362 K and Δ?∘ΔH∘ is positive. A vessel is charged with 48.2 Pa48.2 Pa HBr,HBr, 1430 Pa1430 Pa H2,H2, and 3295 Pa3295 Pa Br2Br2 at 1362 K.
Will the reaction proceed to the left or right to reach equilibrium?
right
left
Calculate the pressure (in pascals) of each species at equilibrium.
?H2=
?Br2=
?HBr=
If the mixture is compressed to half of its original volume, will the reaction proceed to the left or the right to reestablish equilibrium?
left
neither
right
If the mixture is heated from 13621362 to 1421 K1421 K, will HBr be formed or consumed to reestablish equilibrium?
consumed
neither
formed
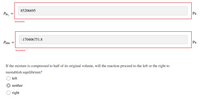
Transcribed Image Text:85206695
PBr2
Pa
Incorrect
-170406751.8
PHB.
Ра
Incorrect
If the mixture is compressed to half of its original volume, will the reaction proceed to the left or the right to
reestablish equilibrium?
left
neither
right
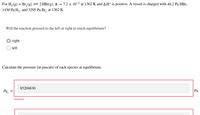
Transcribed Image Text:For H, (g) + Br, (g) =2 HBr(g), K = 7.2 × 10-4 at 1362 K and AH° is positive. A vessel is charged with 48.2 Pa HBr,
1430 Pa H,, and 3295 Pa Br, at 1362 K.
Will the reaction proceed to the left or right to reach equilibrium?
right
left
Calculate the pressure (in pascals) of each species at equilibrium.
85204830
PH,
Pa
Incorrect
Expert Solution
arrow_forward
Step 1
Given :
We have to calculate pressure at equilibrium.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the system SO3(g)SO2(g)+12 O2(g)at 1000 K, K=0.45. Sulfur trioxide, originally at 1.00 atm pressure, partially dissociates to SO2 and O2 at 1000 K. What is its partial pressure at equilibrium?arrow_forwardShow that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation KI(aq)+I2(aq)KI3(aq) give the same expression for the reaction quotient. KI3 is composed of the ions K+ and I3-.arrow_forwardMethanol can be synthesized by means of the equilibriumreaction CO(g)+2H2(g)CH3OH(g) for which the equilibrium constant at 225°C is 6.08103. Assume that the ratio of the pressures of CO(g) and H2(g) is 1:2. What values should they have if the partial pressureof methanol is to be 0.500 atm?arrow_forward
- For the system 2SO3(g)2SO2(g)+O2(g) K=1.32 at 627. What is the equilibrium constant at 555C?arrow_forwardConsider the following system at equilibrium at 25C: PCl3(g)+Cl(g)PCl5(g)G=92.50KJ What will happen to the ratio of partial pressure of PCl5 to partial pressure of PCI3 if the temperature is raised? Explain completely.arrow_forwardWrite the equilibrium constant expression for each reaction in terms of activities, simplifying where appropriate. a C(s)+O2(g)CO2(g) b P4(s)+5O2(g)P4O10(s) c 2HNO2(g)+3Cl2(g)2NCl3(g)+H2(g)+2O2(g)arrow_forward
- What is the approximate value of the equilibrium constant KP for the change C2H5OC2H5(l)C2H5OC2H5(g) at 25 C. {Vapor pressure was described in the previous Chapter on liquids and solids; refer back to this chapter to find the relevant information needed to solve this problem.)arrow_forwardDescribe a nonchemical system that is in equilibrium, and explain how the principles of equilibrium apply to the system.arrow_forwardDistinguish between the terms equilibrium constant and reaction quotient. When Q = K, what does this say about a reaction? When Q K, what does this say about a reaction? When Q K. what does this say about a reaction?arrow_forward
- Explain why the development of a vapor pressure above a liquid in a closed container represents an equilibrium. What are the opposing processes? How do we recognize when the system has reached a state of equilibrium?arrow_forwardThe reaction, 3 H2(g) + N2(g) (g), has the fol lowing equilibrium constants at the temperatures given: atT=25°C,K= 2.8 X 104 at T = 500°C, A = 2.4 X IO"7 At which temperature are reactants favored? At which temperature are products favored? YVhat can you say about the reaction if the equilibrium constant is 1.2 at 127°C?arrow_forwardIf wet silver carbonate is dried in a stream of hot air. the air must have a certain concentration level of carbon dioxide to prevent silver carbonate from decomposing by the reaction Ag2CO3(s)Ag2O(s)+CO2(g) H for this reaction is 79.14 kJ/mol in the temperature range of 25 to 125C. Given that the partial pressure of carbon dioxide in equilibrium with pure solid silver carbonate is 6.23 103 torr at 25C, calculate the partial pressure of CO2 necessary to prevent decomposition ofAg2CO3 at 110C. (Hint: Manipulate the equation in Exercise 79.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning