
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
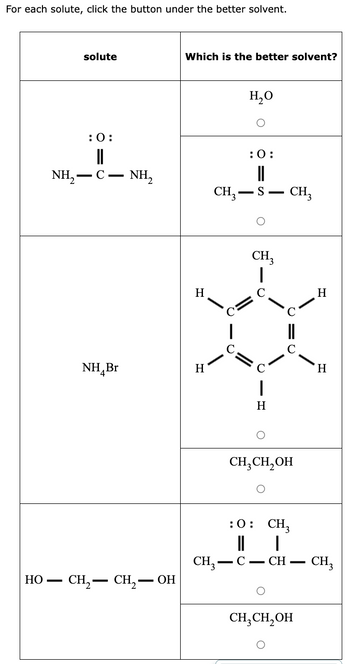
Transcribed Image Text:For each solute, click the button under the better solvent.
solute
:0:
||
NH,−C− NH,
HO-
NH Br
CH₂ - CH₂-OH
Which is the better solvent?
H
H
CH3-
CH₂
H₂O
:0:
||
S
CH3
1
H
CH3
CH₂CH₂OH
:O: CH3
C. - CH-
CH₂CH₂OH
H
H
CH3
Expert Solution
arrow_forward
Step 1
Solubility of a substance in a solvent follows
"Like dissolves like " which means if any solvent is polar then polar substances will be dissolved in it.
Example : NaOH in water.
If the solvent is non-polar then the non-polar substances will be dissolved in it.
Example : hexane in Toulene
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the %(m/v) of hydrochloric acid using the molar concentration you found. Shows calculations. Records final answer to correct significant digits. Records the concentration with the appropriate units. The molar concentration I found was 0.1M.arrow_forwardAnswer both questions pleasearrow_forwardPlease give answer in correct number of sig figsarrow_forward
- The [OH-] of an aqueous solution is 3.5x10 -5. What is the [H+]arrow_forwardCalculate Pharrow_forwardEach row of the table below describes an agueous solution at about 25 °C. [H,0 ]to Complete the table. That is, fill in any missing entries in the second and third columns. Round your entries for to 2 significant digits, and your entries for pH to 2 decimal places. [x,0] solution pH 5.6 x 10 -9 mol/L A O mol/L 7.15 B 0.049 mol/Larrow_forward
- Calculate the pHpH of each solution. 1. [OH−] = 9.9×10−7 M 2.[OH−] = 8.6×10−8 M 3.[OH−] = 3.2×10−11 M 4.arrow_forward3arrow_forwardCalculate either [H3O+] or [OH−] for each of the solutions at 25 °C. Solution A: [OH−]=2.73×10−7 M; [H3O+]= Solution B: [H3O+]=8.31×10−9 M; [OH−]= M Solution C: [H3O+]=6.79×10−4 M; [OH−]= M Which of these solutions are basic at 25 °C? Solution B: [H3O+]=8.31×10−9 M Solution C: [H3O+]=6.79×10−4 M Solution A: [OH−]=2.73×10−7 Marrow_forward
- Choose the correct answer from among the alternatives; To make the sentence true: hydrocyanic acid HCN, the ionization constant Kaq = A) [Ka = [H3O +] / [H2O] [HCN B) [Ka = [H3O +] [CN-] / [HCN C)[Ka = [CN-] / [H2O] [HCN D) [Ka = [CN-] / [H2Oarrow_forward[OH-] = 5.6 × 10-4 M?arrow_forwardCircle the ions that would undergo hydrolysis when added to waterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY