
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
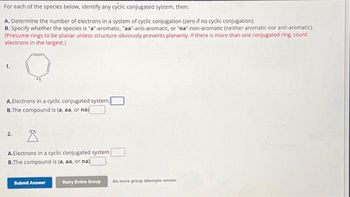
Transcribed Image Text:For each of the species below, identify any cyclic conjugated system, then:
A. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation).
B. Specify whether the species is "a"-aromatic, "aa-anti-aromatic, or "na"-non-aromatic (neither aromatic nor anti-aromatic).
(Presume rings to be planar unless structure obviously prevents planarity. If there is more than one conjugated ring, count
electrons in the largest.)
A.Electrons in a cyclic conjugated system.
B.The compound is (a, aa, or na)
2.
A.Electrons in a cyclic conjugated system.
B.The compound is (a, aa, or na)
Submit Answer
Retry Entire Group
No more group attempts remain
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OChem Help... Label the following as aromatic, antiaromatic or nonaromatic. Next, show how Huckel's rule is applied in each case. See attached photo.arrow_forwardD. Nomenclature of Arenes (Benzenoid aromatics only). Benzene is a six-membered ring compound with alternating double and single bonds. The delocalization of the conjugated double bonds leads to resonance stabilization of these molecules. WS-2.11: Draw the resonance structures of benzene. WS-2.12: Some substituted benzenes get unique names. 1. 2. 3. 4. 5. OH NH₂ 6. 7. 8. OH 9. HO 10. OH OH OH OH OHarrow_forwardPlease helparrow_forward
- Draw the product of this series of reactions. Submit Answer 1. Br₂ 2. 2 equivalents of NaNH₂, 3. Na/NH3 (1) 4. NBS, hv 5. Br₂ • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If a group is achiral, do not use wedged or hashed bonds on it. • If the reaction produces a racemic mixture, draw both stereoisomers. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ● TAYY ? ChemDoodle Retry Entire Group Ⓒ ? Sn [F 8 more group attempts remainingarrow_forward9arrow_forwardAromaticity a. For the molecule below is the ring (as shown - the particular resonance form drawn) aromatic, why or why not? b. Using correct arrow-pushing convention, show the formation of a resonance form of the above structure that is aromatic. What is the charge of the five-membered ring in the main contributing resonance form? C.arrow_forward
- is this aromatic?show steps to verify your answerarrow_forwardI keep getting this question wrong but I’m doing everything correctly.arrow_forwardConsider the structure of the cyclopentadienyl anion. cyclopentadienyl anion Complete the Frost circle (i.e., use the inscribed polygon method) for the anion. Classify the aromaticity of the compound. Aromatic Nonaromatic Antiaromatic Energy Answer Bank 1 1.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY