
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:o ooo
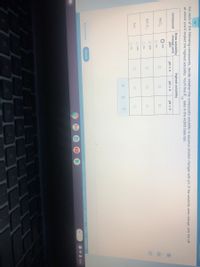
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH
at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab.
sp
Does solubility
change with
pH?
highest solubility
compound
pH = 2
pH = 3
pH = 5
yes
PbCl2
O no
dla
O yes
BaCO,
O no
O yes
ZnS
O no
Explanation
Check
2021 McGraw Hill Education All Rights Rese
Terms of Use
Privacy Accessibility
O VI 1:41
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 10.0 mL of 2.0 103 M Cr(NO3)3 is added to 10.0 mL of a pH = 10.0 NaOH solution, will a precipitate form?arrow_forwardAssuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product: (a) Ag2SO4. (b) PbBr2. (c) AgI. (d) CaC2O4H2Oarrow_forwardA 3.20-L solution of 1.25 103 M Pb(NO3)2 is mixed with a 0.80-L solution of 5.0 101 M NaCl. Calculate Q for the dissolution of PbCl2. No precipitate has formed. Is the solution supersaturated, saturated, or unsaturated?arrow_forward
- Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that changes in the initial concentrations of the common ions can be neglected. (a) TlCl(s) in 1.250 M HCl. (b) PbI2(s) in 0.0355 M Cal2. (c) Ag2CrO4(s) in 0.225 L of a solution containing 0.856 g of K2CrO4. (d) Cd(OH)2(s) in a solution buffered at a pH of 10.995arrow_forwardTwo drops of indicator HIn (Ka = 1.0 109), where HIn is yellow and In is blue, are placed in 100.0 mL of 0.10 M HCl. a. What color is the solution initially? b. The solution is titrated with 0.10 M NaOH. At what pH will the color change (yellow to greenish yellow) occur? c. What color will the solution be after 200.0 mL NaOH has been added?arrow_forwardSketch the titration curve for a weak acid titrated by a strong base. When performing calculations concerning weak acidstrong base titrations, the general two-slep procedure is to solve a stoichiometry problem first, then to solve an equilibrium problem to determine the pH. What reaction takes place in the stoichiometry part of the problem? What is assumed about this reaction? At the various points in your titration curve, list the major species present after the strong base (NaOH, for example) reacts to completion with the weak acid, HA. What equilibrium problem would you solve at the various points in your titration curve to calculate the pH? Why is pH 7.0 at the equivalence point of a weak acid-strong base titration? Does the pH at the halfway point to equivalence have to be less than 7.0? What does the pH at the halfway point equal? Compare and contrast the titration curves for a strong acidstrong base titration and a weak acidstrong base titration.arrow_forward
- Sketch a pH curve for the titration of a weak acid (HA) with a strong base (NaOH). List the major species, and explain how you would go about calculating the pH of the solution at various points, including the halfway point and the equivalence point.arrow_forward. The solubility product of iron(III) hydroxide is very small: Ksp=41038at 25 °C. A classical method of analysis for unknown samples containing iron is to add NaOH or NH3. This precipitates Fe(OH)3, which can then be filtered and weighed. To demonstrate that the concentration of iron remaining in solution in such a sample is very small, calculate the solubility of Fe(OH)3in moles per liter and in grams per liter.arrow_forward12.109 Copper(II) iodate has a solubility of 0.136 g per 100 g of water. Calculate its molar solubility in water and its Ksp.arrow_forward
- A 65.0-mL sample of 0.010 M Pb(NO3)2 was added to a beaker containing 40.0 mL of 0.035 M KCl. Will a precipitate form?arrow_forwardSilver(I) sulfate (Ksp=1.2105) is used in the electroplating of silver. A 1.0-L solution is prepared by mixing 15 g of silver nitrate with 20 g (2 significant figures) of potassium sulfate. Will a precipitate form? At what [Ag+] concentration will precipitation (if any) start?arrow_forwardA saturated solution of lead iodate in pure water has an iodate-ion concentration of 8.0 105 M. a What is the molar solubility of lead iodate in a 0.15 M lead nitrate solution at the same temperature? b Should the molar solubility of lead iodate in part a be the same as, greater than, or less than that of lead iodate in pure water? Why?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning