
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
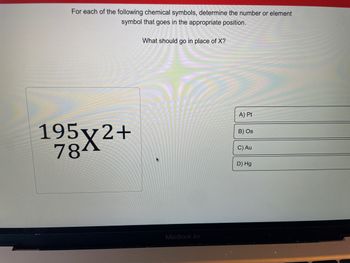
Transcribed Image Text:For each of the following chemical symbols, determine the number or element
symbol that goes in the appropriate position.
195x2+
784
What should go in place of X?
MacBook Air
A) Pt
B) Os
C) Au
D) Hg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which one of the following would have approximately the same mass as 40.0 cm³ of lead (d = 11.3 g/cm³)?arrow_forwardWhich pair of elements have the most similar chemical properties? Question 1 options: Na & K Si & Ne N & Farrow_forwardStates of matter are distinct forms that different phases of matter take on. How many physical state changes there are? O 3 Esc O 1 73°F Partly sunny F1 O 7 Q 4 2 F2 2 W F3 #3 E B $ 4 F5 R % er de F6 丛 T Search F7 Y Y F8 & 7 H F9 * 00 8 F10 128 9 ¢ Ο « F11 K Prt Sc [ Insert Backspace 5:44 P 1/28/202 7 не Enterarrow_forward
- Complete each row of the table below by filling in the missing prefix or missing exponent. 1 IL - 9 10 L = 1 10 1 c L 10 1 d L 10 L =arrow_forwardComplete each row of the table below by filling in the missing prefix or miss 1 J 10 J = 1 m J 10 J -6 J 10 J 1 dJ 10 Jarrow_forward國 ① 14 14 1. 6C 7N+ 14 1N 1H 0. le 0. -learrow_forward
- Please see imagearrow_forwardAn alien from the Planet X uses the units below for mass. How many zigs is 2.5 hums? 3 zigs = 1 cog 4 bips = 27 hums 1 bip = 8 cogsarrow_forwardAlumimnum foil dimensions Length (cm)14.45 cm Width (cm)11.41 cm Mass of aluminum foil (g) 8.593 g What is the volume of the aluminum foil (cm^3)? What is the thickness of the aluminum foil (cm)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY