
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
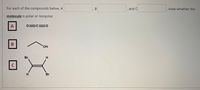
Transcribed Image Text:For each of the compounds below, A
В
and C
state whether the
molecule is polar or nonpolar.
O=C=o
B
HO,
Br
C
Br
H.
Expert Solution
arrow_forward
Step 1
A) The given molecule is,
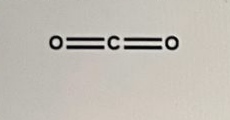
The given molecule is non-polar.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D H chemical symbol, chemical formula or Lewis structure H | HC H | N H- HIC - C C - H Η H HIC C I | H H — — - H Cr O-H Ar :O: O HIC - H C H - H - X boiling point (Choose one) ◊ (Choose one) (Choose one) (Choose one) ✪ Sarrow_forwardAre the structures below polar or nonpolar? Circle the polar ones. Explain why the are polar and indicate the direction of all polar bonds.arrow_forwardEvery atom in the molecule below shows a correct bonding pattern (free electron pairs are not shown). The molecule also contains at least 2 Cs and at bond: // H-C-C | ОН O True O False エーOーエarrow_forward
- Consider a molecule with the following Lewis structure: What is the approximate O-A-O angle (denoted 0)? O 0 = 180° O 120° 00arrow_forwardEffect of Differences in Electronegativity on Molecular Polarity For each molecule: Sketch each molecule as shown in the simulation. Include arrows to show the bond dipoles as well as a molecular dipole (if present). Circle polar or nonpolar to indicate the polarity of the molecule. Fill in the electronegativity values for each atom C. Oz vs HF O2 HF polar Electronegativity nonpolar polar nonpolar H=D F = Question 2a: Both of these molecules have the same molecular geometry; they are linear. How do differences in electronegativity affect the molecular polarity in linear molecules? Page 8 of 9 CHM CZ1 ONL Genmetru chrt p-ts voih 1032 Corvrinht e 2020-2021 B CSI Aarrow_forwardWhich of the following is nonpolar? O NO2 SO3 PH3 HCN CHCI3arrow_forward
- C5H12 What is the electron geometry around each C? Does the geometry around any of the carbon atoms change from structure to structure? Does the overall shape of the molecule change? Describe these changes.arrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name compound hypochlorous acid hydrogen fluoride methanimine formula or Lewis structure HCIO HF H 1 H-C=N-H Between molecules of the compound? O yes O no O yes O no hydrogen-bonding force O yes O no Between molecules of the compound and molecules of water? O yes O no O yes Ono O yes O no X Garrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name methane formic acid compound hydrogen fluoride formula or Lewis structure CHA :0: 11 H-C-O-H HF Between molecules of the compound? O yes O no O yes O no hydrogen-bonding force O yes O no Between molecules of the compound and molecules of water? O yes O no yes O no O yes O no X Garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY