
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
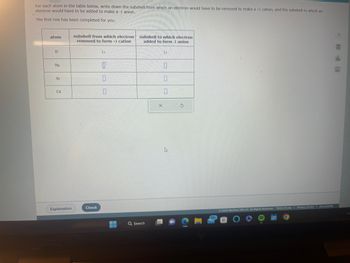
Transcribed Image Text:For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an
electron would have to be added to make a -1 anion.
The first row has been completed for you.
atom
H
Na
Ar
Cd
Explanation
subshell from which electron
removed to form +1 cation
Check
1s
0
0
▬▬▬▬
subshell to which electron
added to form -1 anion
Q Search
15
10
1
0
X
h
G
99+
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
****
O
BEEN
olo
Ar
Expert Solution
arrow_forward
Step 1
Electronic configuration:
Electronic configuration can be define as the arrangement of electrons systematically into various orbitals around the nuclei in accordance to the energy level of an atom.
• When an atom removes 1 electron it forms +1 cation.
• When an atom adds 1 electron it forms -1 anion.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- lon Commonly Formed Name of element SYMBOL Number of Number of electrons in ion protons in ion fluorine Ga 12 potassium Sarrow_forwardWhich element (X) forms an ionic compound with the formula NagX? © magnesium O sulfur O carbon O lodine O phosphorusarrow_forwardThe addition of one or more electrons to a neutral atom results in a O positively-charged cation negatively-charged anion O negatively-charged cation O positively -charged anion DEarrow_forward
- When sodium reacts with chlorine to form an ionic compound, each metal atom loses _____ electron(s) and each nonmetal atom gains _____ electron(s). There must be ___(1,2,3) sodium atom(s) for every ___ (1,2,3) chlorine atom(s) in the reaction.arrow_forwardFor each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you.arrow_forwardA monatomic ion with a charge of -2 has an electronic configuration of 1s22s22p63s23p64s23d104p65s24d105p6.This ion is a(n) _______cation anion.What is the chemical symbol of the noble gas this ion is isoelectronic with? Xe.What is the formula of the ion? Ba2+.arrow_forward
- For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you. atom H Ca Zn I subshell from which electron removed to form +1 cation 1s 0 0 subshell to which electron added to form -1 anion x 1s 0arrow_forwardDetermine the number of valence electrons for each of the following atoms. Enter each answer as a numeral. For example, if an atom has two valence electrons, enter the numeral 2. а) Вe b) Al с) О d) Ararrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY