
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
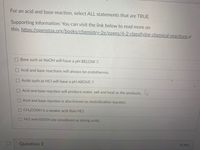
Transcribed Image Text:For an acid and base reaction, select ALL statements that are TRUE.
Supporting information: You can visit the link below to read more on
this. https://openstax.org/books/chemistry-2e/pages/4-2-classifying-chemical-reactions e
Base such as NaOH will have a pH BELOW 7.
Acid and base reactions will always be endothermic.
Acids such as HCI will have a pH ABOVE 7.
OAcid and base reaction will produce water, salt and heat as the products.
O Acid and base reaction is also known as neutralization reaction.
CH3COOH is a weaker acid than HCI.
HCI and H2S04 are considered as strong acids.
4 pts
Question 2

Transcribed Image Text:For the set up of calorimeter, which statement is FALSE?
Thermometer
Precision
thermometer
Insulating cover
Motorized
stirrer
Stirrer
Insulating support ring
Metal inner vessel
Metal outer vessel
(b)
(a)
Supporting information: You can visit the link below to read more on this.
https://openstax.org/books/chemistry-2e/pages/5-2-calorimetry, e
O The stirrer is needed to ensure the solution is mixed well.
O The mass of the solution will be used to calculate the heat (q).
O The thermal energy released by the reaction will be absorbed by the surrounding.
O The thermometer will be measuring the change in temperature of the solution.
O The thermometer will be measuring the change in temperature of the reaction directly.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please answer both questions?arrow_forward[Review Topics] (References) Use the References to access important values if needed for this question. What volume of a 0.101 M HCl solution is required to neutralize 28.5 mL of a 0.150 M KOH solution? HCI+ KOH →KCI + H2O mL HCI Submit Answer Retry Entire Group 9 more group attempts remaining req req Cengage Learning | Cengage Technical Support 98°F Rain coming PrtScn Home F9 PyDn F4 F5 End PyUp F8arrow_forward人工知能を使用せず、 すべてを段階的にデジタル形式で解決してください。 ありがとう SOLVE STEP BY STEP IN DIGITAL FORMAT DON'T USE CHATGPT 2. Determine the concentration of hydrogen ions and hydroxide ions of the following solutions if known: a) pH= 1.5 b) pH = 2.2 c) pOH = 8.8 d) pOH = 9.5arrow_forward
- Guide d Notes (week 3) with an additional part added. 1) Write balanced molecular equations for the neutralization of equal molar amounts of the following acids and bases. Indicate whether the resulting solution after neutralization is acidic, neutral or basic. Provide a written explanation of your choice. a) HOCI and KOH b) HBr and NH3arrow_forwardGive the correct chemical formulas of the products of an acid-base neutralization between H2SO4 and Al(OH)3. (Numbers will be assumed to be subscripts in your answer.) Do not try to enter any physical states or coefficients in the answer boxes. The order in which you list the products does not matter. Formula of one product (do not include the coefficient): Answer Formula of other product (do not include the coefficient): Answer What is the biggest coefficient in the balanced chemical equation for the reaction? (Report a single digit for your answer.) Answerarrow_forwardSodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 559 g NaOH(s) per liter of solution. Calculate the molarity of this saturated NaOH(aq) solution. concentration: M Question Source: MRG - General Chemistryarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY