
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
18. thermodynamics
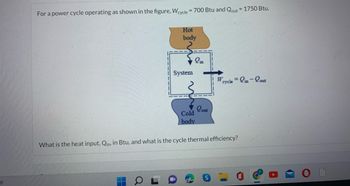
Transcribed Image Text:PF
For a power cycle operating as shown in the figure, Wcycle = 700 Btu and Qout = 1750 Btu.
Hot
body
▬
System
in
Cold
body
in
Lout
W
cycle = in-out
What is the heat input, Qin, in Btu, and what is the cycle thermal efficiency?
Transcribed Image Text:Step 1
What is the heat input, Qin, in Btu, and what is the cycle thermal efficiency?
What is the heat input, Qin, in Btu?
Qin
Cold
body
Btu
Lout
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- At steady state, a power cycle develops a net power output of 20kW while receiving energy by heat transfer at a rate of 20kJ per cycle of operation from a source at a temperature T. The cycle rejects energy by heat transfer to cooling water at a lower temperature of 540 K. If there are 190 (engine thermodynamic) cycles per minute, what is the minimum theoretical value for T, in K?Note: mm is the lastarrow_forward19. thermodynamicsarrow_forwardSteam Transmission Liquid (c) When the steam in the piston-cylinder assembly expands, the transmission converts the piston motion to rotary motion of a paddlewheel that stirs a viscous liquid. The principal source of irreversibility isarrow_forward
- Which of the following principles is the closest interpretation of the first law of thermodynamics? O Energy is continuously and indefinitely discharged more than it receives. O The mass within a closed control volume does not change. O The change of the total energy is equal to the rate if work performed. ON O All real processes tend toward increased entropy. O The net energy crossing the system boundary is the change in energy inside the system.arrow_forwardINTRODUCTION: In thermodynamics, energy can be neither created nor destroyed (1st law): it is either converted to usable work or lost as waste heat. The second law of thermodynamics states that the total entropy (disorder) of a system will never decrease over time; that is, systems can only be at constant entropy or have increasing entropy. This may be restated by saying that there is an upper limit to the efficiency of the conversion of heat to work, as in a heat engine. From these principles of thermodynamics, it is clear that there is an upper limit to how efficient power generation can be at most power plants. Many power plants, including those fueled by fossil fuels, nuclear sources, geothermal sources, biomass, and solar thermal, operate by generating a source of heat (burning fossil fuels, reacting nuclear materials, using the sun’s rays, etc.), using this heat source to heat water to steam, and passing this steam through a turbine. When the turbine spins, power is generated.…arrow_forwardThe larger the specific volume, the greater the work produced (or consumed) by a steady-flow device.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY