
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For a particular reaction at 148.1 °C,
Δ?=−1428.51 kJ, and Δ?=393.69 J/K .
Calculate Δ? for this reaction at −62.8 °C.
Δ?=Δ?−?Δ? thus,
Δ?=Δ?+?Δ?
Assume that enthalpy and entropy will not change between the two temperatures.
Use these values with the new temperature to determine the new free energy value using the first equation.
Expert Solution
arrow_forward
Step 1
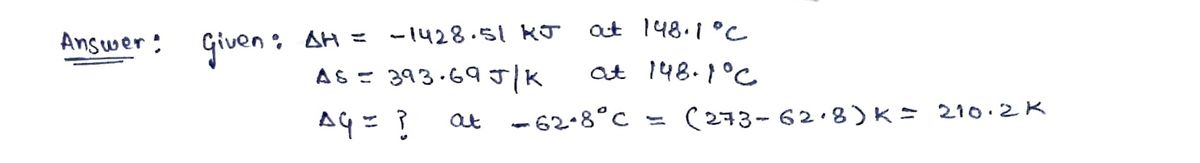
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You discover a new chemical reaction that has a standard entropy change equal to 167.1 J/mol•K and a standard enthalpy change of 60.20 kJ/mol at 296 K. Calculate the change in free energy (in kJ) for this reaction. Report your answer to the tenths place and do not include units. Include the correct sign in your answerarrow_forwardPart 1 and part 2 go together For part 2, Then use the data below to calculate ∆G rxn using free energies of formation.arrow_forwardCalculate for the ΔS given the values of ΔHo = +119.2 kJ, ΔGo= +13.4 kJ at 298.5 K of temperature. CO(NH2)2(aq) + H2O(l) ----> CO2(g) + 2 NH3(g)arrow_forward
- For each of the following processes, indicate whether the signs of ∆S and ∆H are expected to be positive, negative, or about zero. A piece of charcoal is combusted to form CO21g2 and H2O1g2arrow_forwardWhat is entropy, and what is ΔS for a reaction, and what does it mean if ΔS is positive or negative?arrow_forwardFor the reaction 2 A(g) → A2(g), Q = -20.00 kJ for the formation of onemole of A2(g) at constant volume and a temperature of 25.00◦C. If we did thissame reaction at constant pressure, what would be the values of Q, W, ∆U,and ∆H for the formation of one mole of A2(g) at a temperature of 25.00◦C?arrow_forward
- The specific heat capacity of ice is 2.04 J/g⋅∘C and its heat of fusion is -332 J/g. Calculate the q for melting 127 g of ice to 16 degrees C. Find the w for this process. Find ΔE for this process. Find ΔH for this process.arrow_forwardFor a particular reaction at 148.1 °C, delta G=−1428.51 kJ, and delta S =393.69 J/K . Calculate delta G for this reaction at −62.8 °C. Free energy is related to enthalpy, temperature, and entropy through the equation delta G = delta H -T(delta S) Δ?=Δ?−?Δ? delta H = delta G + T (delta S) Solve for the enthalpy of the reaction. Assume that enthalpy and entropy will not change between the two temperatures. Use the values from the prior scenario (temperature) to determine the new free energy value using the first equation.arrow_forwardFor a particular reaction, Δ?=−14.20 kJ and Δ?=−198.5 J/K. Calculate Δ? for this reaction at 298 Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY