
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
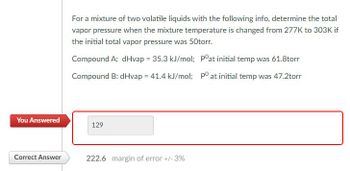
Transcribed Image Text:For a mixture of two volatile liquids with the following info, determine the total
vapor pressure when the mixture temperature is changed from 277K to 303K if
the initial total vapor pressure was 50torr.
Compound A: dHvap = 35.3 kJ/mol; Pᵒat initial temp was 61.8torr
Compound B: dHvap = 41.4 kJ/mol; po at initial temp was 47.2torr
You Answered
129
Correct Answer
222.6 margin of error +/- 3%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- em 119 My Course X Macmillan: X Course Mo Submit Answer X Sections 5 What is the FORMULA for the limiting reagent? References X age.com/static/nb/ui/evo/index.html?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786042&snapsh What amount of the excess reagent remains after the reaction is complete? HOMEWOR Use the References to access Important values if needed for this question. For the following reaction, 20.3 grams of carbon dioxide are allowed to react with 39.6 grams of potassium hydroxide. carbon dioxide (g) + potassium hydroxide (aq) potassium carbonate (aq) + water (1) - What is the maximum amount of potassium carbonate that can be formed? MacBook Air X grams MindTap - grams ;arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- 2+ d. 4H3O+ (aq) + 2Cl(aq) + MnO₂ (s) ⇒ Mn²+ (aq) + 6H₂O(1) + Cl₂ (9) Oke O Ke = = O Ke = 2+ [Mn²+ ][C1₂] [H3O+] * [CI-1² [Mn²+][H₂O][C1₂] 2+ [H³O+][CI¯]²[MnO₂2] [Mn²+ ] [H₂O1] [C1₂] 2+ [H3O+] * [CI-1²[MnO₂]arrow_forwardsession.masteringchemistry.com/myct/itemView?assignment ProblemID=190556628&offset=next Discussion Question 1: Chapter 6 Homework blem 6.20- Enhanced - with Feedback Enter the symbols for the ions and the correct formula for the onic compound formed by each of the following. esc Q A N F1 2 W S F2 X #3 20 E D F3 с S4 $ OOO 000 R Part E F4 F Enter the symbols for the ions of sodium and phosphorus. Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr²+, As³-). cation, anion = Part F Complete previous part(s) Submit Part G cation, anion Submit Part H Complete previous part(s) 65° V Enter the symbols for the ions of calcium and sulfur. Enter the lons formed by these elements and separate your answers with a comma % Request Answer T Request Answer G 6 ΑΣΦ B ΑΣΦ MacBook Air F6 Y H 18⁰ h & 7 N F7 U * CO 8 J BUT RAS poddany ▶II ? F8 1 ? E 9 K F9 O L (e.g., Sr²+, As³-). 0 Ga3+ and 02-E L F10 P F11arrow_forwardEnter the ions present in a solution of K₂CO3. Express your answers as chemical formulas separated by a comma. Offset subscripts and charges on each ion; for charges, write the number before the + or - sign. View Available Hint(s) 5 Provide Feedback Submit esc ΑΣΦ Mother to Son &....pdf A lock ! 1 F1 Q GOOD A @ 2 N 30 F2 W S ? #3 80 F3 X E I дв control option command D A SA 4 Q F4 C R FL O % 5 & F5 T V MacBook Air 6 G F6 Y B & 7 H F7 U N * 8 J DII FB 1 ( 9 M K FO O ) V H 0 < L F10 P commandarrow_forward
- beaded) SIGNATURE Print Staff Name SH w ти department HOLD VOLUME Updat SPEAKER GHI4 $7 PORS TONE a-A NEX OPERO CELL A MIC Type here to search Direct Care CELL B SEN CHROME = ve to Todd CELL CHARGE URL In address bar: odd. my.gov/pm/ehr/training Name: DirectCare017@email.com OPWDDTRAINING Password: test@1234 O NAMING AND DRAWING ORGANIC MOLECULES Interpreting the skeletal structure of a neutral organic molecule Answer the questions in the table below about this molecule: H 017 What is this molecule's chemical formula? Note: write the simplest molecular chemical formula, in which each element symbol appears only once. How many CH3, CH2, and CH groups are in this molecule? Explanation Check Kim Stampone (585) 451-7403 - Social Worker for EH starting tomorrow Nightly Report Distribution List: Perry Vandunk Katie Metz April Rowell TTL DA3 DA3 ly King St Bank St Manle Ave 0 CH, [сн, сн URC Codes for GEN/ORL Homes: 9968 6550 27 0168 00 6119 IdH X 5 3/5 Jessica V ? 000 Ar 8: K Ⓒ2023 McGraw…arrow_forwardHNO3 е. Show Hintarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY