
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
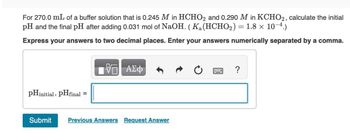
Transcribed Image Text:For 270.0 mL of a buffer solution that is 0.245 M in HCHO2 and 0.290 M in KCHO2, calculate the initial
pH and the final pH after adding 0.031 mol of NaOH. (Ka (HCHO2) = 1.8 × 10-4.)
Express your answers to two decimal places. Enter your answers numerically separated by a comma.
pHinitial, pHfinal
=
ΜΕ ΑΣΦ
Submit
Previous Answers Request Answer
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- For 480.0 mL of a buffer solution that is 0.120 M in HC2H3O2 and 7.50×10−2 M in NaC2H3O2 , calculate the initial pH and the final pH after adding 1.5×10−2 mol of HCl . ( Ka(HC2H3O2)=1.8×10−5 .)arrow_forwardGiven 1.00 L of a solution that is 0.100 M in sodium propionate (NaC3H5O2 ) and 0.300 M in propionicacid (HC3H5O2 ), what is the pH after 0.0400 mole of HNO3 is added? Assume that the volume does notchange upon addition of the HNO 3 . Ka for HC3H5O2 = 1.3 × 10−5arrow_forwardWhat is the pH of a buffer solution that is 0.10 M chloroacetic acie, HC2H2ClO2 and 0.15M sodium chloroacetate NaC2H2ClO2? The Ka for HC2H2ClO2 is 1.4x10-3. Express your answer to two decimal places.arrow_forward
- Calculate the pH of a titration solution formed when 45.0 mL of 0.100 M NaOH is added to50.0 mL of 0.100 M CH3COOH (Ka = 1.8×10-5).arrow_forwardWhat is the pH of a solution prepared by combining 0.258 mol of a newly discovered weak acid (Ka = 1.3 x 10-4) with 0.156 mol of the salt containing its conjugate base in 1.4 L of water?arrow_forwardA 15.0 mL sample of 0.25 M NH3 is titrated with 0.10 M HCl at 25 oC. What is the pH of the resulting solution when 19.50 mL of titrant have been added?Enter your answer with two decimal places. At 25 oC: Kb for NH3 = 1.8 x 10-5arrow_forward
- Q2.9 What would be the pH of a buffer that is composed of mh of 0.10 M CH3 CaoNa? and 125 92 mL of 0.10M CH3COOH known: Ka (CH3 COOH) = 1.81x10-5 write. your answer to 2 decimal places.arrow_forwardYou need to prepare a buffer solution from NaH2 PO4 and Naz HPO4 to have a pH of 8.00. What ratio of amounts of Naz HPO4 and NaH2PO4 should you take? (Ka2 = 6.2 × 10-8) (Simplify your answer completely.) n(Naz HPO4) n(NaH2 PO4)arrow_forwardThe Ka of lactic acid is 1.38 x 10^-4arrow_forward
- NaOH is added to 0.040 M A1³+, raising the pH to 13.60. which is the predominant species: Al(OH)3 or Al(OH)Ã? (K for Al(OH)² O Al(OH)3 O Al(OH) O They will be equal. 3=1.8 × 10-33, K, for Al(OH)4 = 2.0 × 10³³) Select the single best answer.arrow_forwardWhat is the approximate pH at the equivalence point of a weak base-strong acid titration if 50.00 mL of aqueous ammonia (NH3) requires 37.10 mL of 0.1087 mol L-1 HCI? pK₁ =4.75 for ammonia. Report your answer in 2 decimal places.arrow_forwardWhat is the pH of a solution containing a mixture of 0.50 M HCN and 0.040 M NaCN? (Ka = 4.9 x 10-10 for HCN ).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY