
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
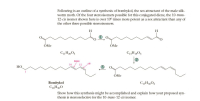
Transcribed Image Text:Following is an outline of a synthesis of bombykol, the sex attractant of the male silk-
worm moth. Of the four stereoisomers possible for this conjugated diene, the 10-trans-
12-cis isomer shown here is over 106 times more potent as a sex attractant than any of
the other three possible stereoisomers.
H
H
ÓMe
ÓMe
trans
12
cis
HO
(3)
ÓMe
Bombykol
C16H3,0
Show how this synthesis might be accomplished and explain how your proposed syn-
thesis is stereoselective for the 10-trans-12-cis isomer.
Expert Solution
arrow_forward
Step 1
In the first step, a Wittig reagent with an anion stabilizing group will give E alkene and the formed double bond is conjugated to the aldehyde group in the product. In the second conversion, an alkyl Wittig reagent is used which produces predominantly the Z product. In the las t reaction, lithiumaluminium hydride followed by hydrolysis reduces the ester group to the alcohol group.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Provide a mechanism and explain the stereochemistry at position 3 and 4 of the product. TIPS OBn 3 CHO CS2CO3 + CH3 NO2 EtOH, 23 °C, 90 min TIPS (45:55 dr) (3R,4S)-9 10 OH OH OBn CH3 18 32% isolated yieldarrow_forwardplease,don't provide handwriting solution.arrow_forward9A Give the product(s) of the following reactions according to the mechanism given below the arrow of each reaction. Give the stereochemistry of the product where appropriate EtOH E1 H₂O E1 CI CH 3 -H CH3 X CH3 'CI EtONa E2 H₂O SN1arrow_forward
- Identify the expected major products of the following intramolecular Diels-Alder reaction. CH₂O O CH3O CH₂O OCH3 11 CH₂0 OCH3 En OCH3 CH3O +En IV A CH3O O V ? CH3O сноя Росто OCH3 OCH3 VIarrow_forwardHeating the following molecule in ethanol gave a major product with molecular formula C,H12 , which when treated with O3 followed by Me2S produced the diketone shown. Br CH3CH,OH 1. O3 C,H12 heat 2. Me,S (i) Deduce the structure of the product with molecular formula C,H12 from the information provided. Write an arrow formalism reaction mechanism to show how the compound with molecular formula C,H12 is formed. (ii) How would you perform the following transformation to obtain the final product as the MAJOR product. More than one step is needed. Show all the steps in your synthesis. Reaction mechanisms are not required. Br more than one step less than four steps OHC CHOarrow_forward6. HO3S Synthesize the following compounds starting from benzene: H3C CH3 NH2 Br SO 3H H₂C CH3 Cl CH3arrow_forward
- The reaction of butan-2-ol with concentrated aqueous HBr goes with partial racemization, giving more inversion than retention of configuration. Propose a mechanism that accounts for racemization with excess inversionarrow_forward13. On treatment with nitrous acid (HNO2) these 4 isomers of 1 react differently. (a) Draw a mechanism for the reaction of each stereoisomer, and hence identify which stereoisomer gives which product A, B or C. (b) Draw the orbital interactions involved in the product-determining step for each reaction. A H B C To get you started: OH OH HNO2 1 NH2 NENarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY