
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Fluidization of a Sand Bed Filter. To clean a sand bed filter it is fluidized at
minimum conditions using water at 24"C. The round sand particles have a
density of 2550 kglm3 and an average size of 0.40 mm. The sand has the
properties given in Table 3.1-2.
(a) The bed diameter is 0.40 m and the desired height of the bed at these
minimum fluidizing conditions is 1.75 m. Calculate the amount of solids
needed.
(b) Calculate the pressure drop at these conditions and the minimum velocity for
fluidization.
(c) Using 4.0 times the minimum velocity, estimate the porosity and height of the
expanded bed.
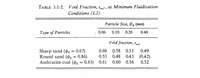
Transcribed Image Text:Void Fraction, ɛmf, at Minimum Fluidization
Conditions (L2)
ТАBLE 3.1-2.
Particle Size, Dp (mm)
Туре of Рarticles
0.06
0.10
0.20
0.40
Void fraction, ɛEms
0.60
0.58
Sharp sand (os = 0.67)
Round sand (Øs = 0.86)
Anthracite coal (os = 0.63) 0.61
0.53
0.49
(0.42)
0.52
0.53
0.48
0.43
0.60
0.56
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 2. A 3-D printer was used to fabricate a porous cartilage scaffold using a hydrogel solution. The hydrogel (viscosity was 3 x 10 Pa-s) was added to the extruder, and a pressure of 5 bar was applied to extrude 500 mL of the solution over 10 minutes to form the desired shape. The needle attached to the extruder had a length of 50 mm and an inner diameter of 1.0 mm. Determine the pressure drop needed to complete this fabrication process.arrow_forward1. During a carburization process for the surface treatment of steel, how much processing time in minutes is needed to increase the carbon content at 0.4% up to 0.85% at (a) 0.5 mm and (b) 1 mm below the surface? Assume carbon content at the surface is 0.95 % and steel inside has background carbon of 0.1% and diffusivity is 1.1 x 10-13 m²/s.arrow_forwardImpermeable. barrier Skin surface Drug patch Infected body tissue (sink for drug) -Drug reservoir -Gel diffusion barrierarrow_forward
- 21. A plate and frame filter press is used to filter a compressible sludge (S = 0.45) at 50 psia for 2 hours. Washing is done at 30 psi with wash water equal to 10% of the filtrate volume collected. The washing time is a. 100 min b. 127 min c. 85 min d. 205 minarrow_forwardExplain the difference (in concept or definition) between boundary layer thickness and thermal boundary layer thickness?arrow_forward2. A 1 L, 100 g (dry weight) sample is removed from a continuous crystallizer which is operated with a residence time of 1000 s. The following particle size distribution is obtained for the sample: i) Size range (um) Mass (g) 200- 300- 400 500 600-700- 800- 300 400 500 600 700 800 900 0- 100- 100 200 2.5 26.0 30.0 21.0 12.0 6.0 2.0 0.5 0.0 Microscopy based on the sieve fractions has found that a volumetric shape correction factor, ky, of 0.52, for the crystal population and the crystal density has been found to be 2000 kg/m³ Determine whether the MSMPR model describes the system. If it does so, then determine the growth and nucleation rates for this system; if it does not, suggest why reasons that may cause deviation of this CSTR type crystallizer from the MSMPR model.arrow_forward
- Provide an example of a mass transfer problem in which both diffusion and convection are important.arrow_forwardb) A complete drying experiment for two different materials (sand and minced meat) was conducted in the laboratory. The results of drying rate for both materials are as shown in Figure 3. Compare and explain the drying rate behavior for sand and minced meat. Drying rate Sand Minced meat Drying time Figure 3 Relation between drying rate and drying timearrow_forwardQ13. The wear resistance of a steel gear is to be improved by hardening its surface. This is to be accomplished by increasing the carbon content within an outer surface layer as a result of carbon diffusion into the steel; the carbon is to be supplied from an external carbon-rich gaseous atmosphere at an elevated and constant temperature. The initial carbon content of the steel is 0.20 wt%, whereas the surface concentration is to be maintained at 1.00 wt%. For this treatment to be effective, a carbon content of 0.60 wt% must be established at a position 0.75 mm below the surface. Specify an appropriate heat treatment in terms of temperature and time for temperatures between 900 °C and 1050 °C (you may select any 3 temperatures). For the diffusion of carbon in steel, Do = 2.3 x 10 m/s, Q = 148 kJ/mol. (The error function values are given below) erf(2) erf(z) 0.55 0.60 1.3 1.4 0.5633 0.9340 0.025 0.0282 0.6039 0.9523 0.05 0.0564 0.65 0.6420 1.5 0.9661 0.10 0.1125 0.70 0.6778 1.6 0.9763…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The