
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Solve this problem step by step
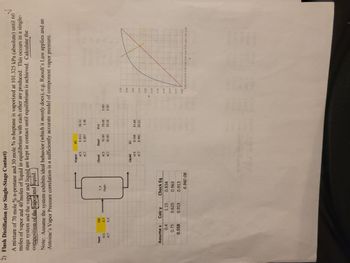
Transcribed Image Text:2) Flash Distillation (or Single-Stage Contact)
60
A mixture of 70 mole % n-pentane and 30 mole % n-heptane is vaporized at 101.325 kPa (absolute) until
moles of vapor and 40 moles of liquid in equilibrium with each other are produced. This occurs in a single-
stage system and the vapor and liquid are kept in contact until equilibrium is achieved. Calculate thell
composition of the vapor and liquid.
Note: Assume the system exhibits ideal behavior (which it mostly does), e.g. Raoult's Law applies and an
Antoine's Vapor Pressure correlation is a sufficiently accurate model of component vapor pressure.alloo
Feed
nC5
nC7
100
0.7
0.3
Assume x Calc y
0.4
0.75
0.558
1.15
0.625
0.913
4
T, P
Flash
Check Eq
0.834
0.963
0.913
6.94E-08
Vapor
nC5
nC7
Check
nC5
nC7
Liquid
nC5
nC7
40
0.913
0.087
Feed
70.00
30.00
60
0.558
0.442
36.52
3.48
V+L
70.00
30.00
33.48
26.52
XELU.O
0080.0
1.0 $0800.0 8038.
0.00
0.00
TACOLO
1.00
0.90
0.80
0.70
0.60
0.50
0.40
0.30
0.20
0.10
0.00
0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00
NA
P080.0
0092.0
0020.0
0000.0
0080.0
20210 0010.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- What if the amount of air is not stated? How can we assume for that? Is there any technique?arrow_forwardFind the residence time for a 10 µm diameter water particle in the atmosphere. Assume the density of the particle is 10 g/m³. The elevation is 1000 m. Repeat the calculation for a 2.5 µm particle.arrow_forwardIn the McCabe–Thiele method, are the stages stepped off from the top down or the bottom up? In either case, when is it best, during the stepping, to switch from one operating line to the other?Why?arrow_forward
- Have post part A and B please show and indicate step and answerarrow_forwardWhat is the difference between Reynolds analogy and the Chilton–Colburn analogy? Which is more useful?arrow_forwardGive a table showing classification of mass transfer operations used in Chemical industries with phases of contactarrow_forward
- 2. In order to remember the difference between macroscopic and molecular scale energies, I ... Answer:arrow_forwardDoes a two-section cascade overcome this limitation?arrow_forward7.40. The cart in Fig. 7.38 has a mass of 2000 kg. It is resting on frictionless wheels on a solid, level surface and encounters no air resistance. At time zero it is standing still, and a jet from a fire hose is used to start it moving. The mass flow rate of the fluid from the fire hose is 100 kg / s, and its velocity relative to fixed coordinates is 50 m/s. The cup on the rear of the cart turns the jet around so that it leaves in the -x direction with the same velocity relative to the cart with which it entered. Calculate the velocity-time behavior of the cart; assume the jet is unaffected by gravity. (This is not a very practical problem, but it is analogous to the more complex and interesting problem of starting a large turbine from rest. All such turbines must be occasionally shut down for maintenance; their starting and stopping behavior is more complex than their behavior running at a steady speed.)arrow_forward
- . (Grinding Operations and Grinding Machines) (USCS units) In a centerless grinding operation, the grinding wheel diameter = 8 in, and the regulating wheel diameter = 5.0 in. The grinding wheel rotates at 1500 rev/min, and the regulating wheel rotates at 180 rev/min. The inclination angle of the regulating wheel = 2.3°. Determine the production rate of cylindrical work parts whose diameter = 0.5 in and length = 5.0 in. Solutions used; fr = TD₂N, sin Iarrow_forwardUnder what conditions is a membrane cascade of multiple stages in series necessary?arrow_forwardGive a brief description of the use of hydrostatic pressure in level measurement. List the main advantages and disadvantages. What is the formula used to deduce level from pressure?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The