
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
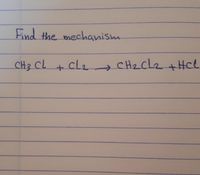
Transcribed Image Text:Find the mechanism
CH3 CL
cl2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Label the components of an energy diagram for a spontaneous reaction. Answer Bank uncatalyzed reaction products activation energy reactants catalyzed reaction Reaction progress–arrow_forwardWhat is the correct Kc expression for the reaction below? HNO3 (1) + CIF(g) = CIONO2(g) + HF(g) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. [HNO3][CIF] [CIONO;][HF] a [CIF] b K. [CIONO,][HF] (CIONO2][HF] (CIF] [CIONO2][HF] [HNO3][CIF] darrow_forwardConsider the reaction. k₁ S P k₂ What effects are produced by an enzyme on the general reaction? AG for the reaction increases. The formation of the transition state is promoted. The reaction equilibrium is shifted toward the products. The concentration of the reactants is decreased. The activation energy for the reaction is lowered. The rate constant for the forward reaction (k₁1) increases.arrow_forward
- 6. What is the AH of the reaction KS K+S7 K+S KS AH = +32.5 kJ KS+ K+ K,S AH = +38.2 kJ O +6 kJ O -32.5 kJ O +70.7 kJ +32.5 LJarrow_forward*54. A compound called di-t-butyl peroxide [abbreviation DTBP, formula (CH3);COOC(CH3)3] decomposes to give acetone [(CH3),CO] and ethane (C,H,): (CH3); COOC(CH3)3 (g) → 2 (CH;)2CO(g) + C2H6 (g)arrow_forward10:07 M ll 93%1 Question 23 of 50 Submit Construct the expression for Kc for the following reaction. 2 C,H,(g) + 02(g) =2 CH;CHO(g) 1 Drag the tiles into the numerator or denominator to form the expression. Ko %3D 5 RESET [C,H,] 2[C,H] 4[C,H,] [C,H,]? [C,H,]4 [0,] 2[0,] 4[0,] [0,]2 [0,]* [CH,CHO] 2[CH,CHO] 4[CH,CHO] [CH,CHO]? [CH,CHO]4 IIarrow_forward
- Check AlISwer < Question 7 of 10 Draw the amide formed when 1-methylethylamine (CH, CH(CH, )NH,) is heated with each carboxylic acid. Reaction A Select Draw Rings More Erase C H CH,CH,CH,COOH + CH,CH(CH, )NH, Reaction B hp end ort sc delete bome 144 num Jock backspace & 24 4 8 T. Y U home R enter 4. J L F pause ↑ shift 1 end V N alt ctrlarrow_forwardConsider the reaction R P. Check all of the following that are true when a catalyst is added to the reaction mixture. The Ea for R P decreases. The value [P] / [R] increases .The rate of reaction P R increases. The rate of reaction R P increases.arrow_forwardRunning the reaction at a high temperature, draw a box around the major product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY