
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
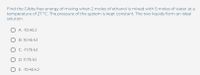
Transcribed Image Text:Find the Gibbs free energy of mixing when 2 moles of ethanol is mixed with 5 moles of water at a
temperature of 27 °C. The pressure of the system is kept constant. The two liquids form an ideal
solution.
O A. -10.45 J
O B. 10.45 kJ
O C. -11.75 kJ
O D. 11.75 kI
O E. -10.45 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- If the value of mass doubles but the volume stays the same, the value for density will bearrow_forwardDescribe the preparation of the following solutions include all mathematic calculations and equipment of the laboratory needed A.500 mm of a solution of KNO 1.50 M B. 150 g of a solution 5.0% of NaC2H3O2 C. 500 mm of a solution of NaOH 0.50 M from a solution of NaOH 6.0 Marrow_forwardWhat is the pH of a cleaning solution with a [H3O*] = 7.4 x 10-10 м НзО"? А. 7.4 В. 7.13 C. 8.13 D. 9.00 E. 9.13arrow_forward
- How many mg in 1.35 g ? Report your answer to 3 decimal places (examples 1.042, 0.050, 0.198, 3.000).arrow_forwardConsider a buffer solution that contains 0.55 M NH2CH2CO2H and 0.35 M NH2CH2CO2Na. pKa(NH2CH2CO2H)=9.88. a. Calculate its pH. b. Calculate the change in pH if 0.155 g of solid NaOH is added to 250 mL of this solution. c. If the acceptable buffer range of the solution is ±0.10 pH units, calculate how many moles of H3O+ can be neutralized by 250 mL of the initial buffer.arrow_forwardWhat is the concentration in % (w/v) of a solution prepared from 50.0 g NaCl and 2.5 L of water? A. 5.0 % B. 2.0 % O C. 0.020 % D. 0.050 % E. 0.50 %arrow_forward
- Nonearrow_forwardCount the significant digits in each of these measurements: number of measurement significant digits ? 82300. kg 0.005100 J - 3.0 x 10 -2 kJ/mol -1 3.1 x 10 mLarrow_forwardWhat is the concentration in % (w/w), of a solution prepared by mixing 50.0 g NaCl and 150.0 g water? A. 0.250 % B. 33.3 % C. 40.0 % D. 25.0 % E. 3.00 %arrow_forward
- 5arrow_forwardIf 46.5 mol of an ideal gas occupies 95.5 L at 373 K, what is the pressure of the gas? pressure: I atmarrow_forwardYou are asked by the pharmacist to add 45 mEq of Ca Gluconate in an IV bag of D5W 1000mL. You have a concentrated vial of Ca Gluconate 4.4 mEq/mL, 50 mL. How many mL of this concentrated vial needs to be added to the IV bag?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON