
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
There is a colloid in which diameter 300 nm particles are dispersed in water. Find the distance traveled by Brown diffusion(x=(2Dt)^1/2 ) and the distance traveled by sedimentation here, and tell me which is the main behavior between sedimentation and diffusion.
here, D=1.0*10^-12m^2/s And particle density is 2.5 g/cm^3
There is stoke equation in picture.
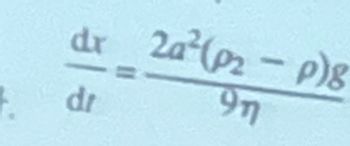
Transcribed Image Text:dr
dr
20²(02-p)8
97
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 13 images

Knowledge Booster
Similar questions
- 7arrow_forwardWhat is the diffusion coefficient of a membrane-bound protein of molar mass 79,300 daltons at 37°C if the viscosity of the membrane is 1 poise (0.10 N.s.m-2)? Assume that this protein is an unhydrated, rigid sphere that has a density of 1.4 g.cm-3. 4.0 81.17e-15 X m².s-1 What is the average distance traveled by this protein in 2.3 s? 4.0 1.058e-8 X 19000 Åarrow_forwardProvide an example of a mass transfer problem in which both diffusion and convection are important.arrow_forward
- Sketch two graphs, the first for a solid that's solubility is increasing as a function of temperature, and the second for a solid whose solubility decrease as a function of temperature. Be sure to label each axisarrow_forwardConsider a diffusion process in a semi-infinite solid with a source at x=0 mm, the concentration of the diffusing species is 2 nm-3 at x=5 mm and t=1 hr (since the start of the process). At what time the concentration at x=15 mm will reach 3 nm-3 ?arrow_forward5arrow_forward
- during a steady state uniolecular diffusion of A through a stagnant film of B, the flux of A will a. increase linearly with z b. decrease linearly with z c. be independent of zarrow_forwardProblem #1: Diffusion-convection problems wherein the species diffusivity is concentration-dependent often provide a differential equation of the following general form: where Cis the dimensionless concentration and z is the dimensionless distance. Assume vL/D, is a constant. Assume that C= 1 at z = 0, and C=0 when z is very large. Solve the above differential equation and provide an exact analytical equation for C=ft:). Problem #2: In a diffusion-reaction system, reactant 'A' diffuses through a porous catalyst pellet and reacts in a 1* order reaction to produce 'B'. Efficacy of the catalyst in converting 'A' to 'B' is a function of 'x' such that the mass balance equation describing the system at steady-state is: d?c Dari -kīC = 0 Cis the concentration of 'A' that varies in the x-direction, k is the 1t order reaction rate constant, and D is the diffusion coefficient of 'A'. L is the thickness of the catalyst layer, and can be treated as a constant. First, non-dimensionalize the…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The