
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Reference table provided
![Find the concentration of I in 0.050 M AgNO3 saturated with AgI. Include activity coefficients in the solubility-product
expression. The K sp of AgI is 8.3 × 10-¹7. Refer to the table of activity coefficients at various ionic strengths as needed.
[r] =
M](https://content.bartleby.com/qna-images/question/af8dd5bb-1368-4a10-9929-9294802d0b74/337bc752-a156-4f6e-b66e-33e1a5135d0f/kkilkr_thumbnail.png)
Transcribed Image Text:Find the concentration of I in 0.050 M AgNO3 saturated with AgI. Include activity coefficients in the solubility-product
expression. The K sp of AgI is 8.3 × 10-¹7. Refer to the table of activity coefficients at various ionic strengths as needed.
[r] =
M
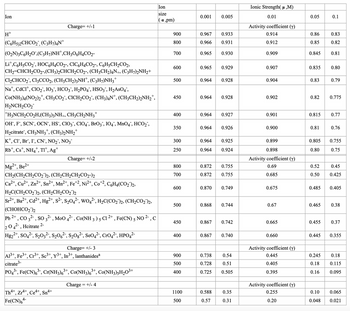
Transcribed Image Text:Ion
H™
Charge= +/-1
(C6H5)2CHCO2, (C3H7)4N+
(O₂N)3C6H₂O,(C3H7)NH*,CH3O6H4CO2-
Lit,C6H5CO₂, HỌC6H4CO2-, C¹C6H4CO2-, C6H5CH₂CO2,
CH₂=CHCH₂CO2-,(CH3)2CHCH₂CO2-, (CH3CH2)4N+, (C3H7)2NH2+
Cl₂CHCO2, C13CCO2, (CH3CH₂)3NH¹, (C3H7)NH3+
Nat, CdC1, C1O₂, 103, HCO3, H₂PO4, HSO3, H₂AsO4",
Co(NH3)4(NO₂)2, CH3CO₂, CICH₂CO₂¯, (CH3)4N*, (CH3CH₂)2NH₂*,
H₂NCH₂CO₂
*H3NCH₂CO₂H,(CH3)3NH+, CH3CH₂NH3+
OH, F, SCN, OCN¯, HS¯, ClO3¯`, C1O4¯, BrO3¯, IO4¯`, MnO4, HCO₂,
H₂citrate", CH3NH3*, (CH3)2NH₂+
K, Cl, Br, I, CN, NO₂, NO3
Rbt, Cst, NH4+, TI+, Ag+
Charge= +/-2
Mg2+, Be2+
CH₂(CH₂CH₂CO2)2, (CH₂CH₂CH₂CO2-)2
Ca²+, Cu²+, Zn²+, Sn²+, Mn²+, Fe+2, Ni²+, Co2, C6H4(CO₂)2,
H₂C(CH₂CO2)2, (CH₂CH₂CO2)2
Sr²+, Ba²+, Cd2+, Hg2+, S²-, S₂04²-, WO4², H₂C(CO₂)2, (CH₂CO₂)2,
(CHOHCO₂)2
2+
Pb 2+, CO 3²-, SO 3²-, MoO 4²-, Co(NH 3 ) 5 C1 ²+, Fe(CN) 5 NO ²-,c
2 04²-, Hcitrate 2-
2+
Hg₂+, SO4²-, S₂O32-, $₂06²-, S₂Og²-, SO4²-, CrO4²-, HPO4²-
Th4+, Zr4+, Ce4+, Sn4+
Fe(CN)64-
Charge= +/- 3
Al³+, Fe3+, Cr³+, Sc³+, y³+, In³+, lanthanidesa
citrate 3-
PO4³-, Fe(CN)6³-, Cr(NH3)6³+, Co(NH3)6³+, C0(NH3)5H₂O³+
Charge = +/- 4
Ion
size
(a ,pm)
900
800
700
600
500
450
400
350
300
250
800
700
600
500
450
400
900
500
400
1100
500
0.001 0.005
0.967 0.933
0.966
0.931
0.965 0.930
0.965
0.964 0.928
0.964
0.964
0.964
0.964
0.964
0.872
0.872
0.868
0.867
0.929
0.738
0.728
0.725
0.928
0.588
0.57
0.927
0.870 0.749
0.926
0.925
0.924
0.755
0.755
0.744
0.867 0.740
0.742
0.54
0.51
0.505
0.35
0.31
Ionic Strength(μ,M)
0.01
Activity coefficient (y)
0.914
0.912
0.909
0.907
0.904
0.902
0.901
0.900
0.899
0.898
Activity coefficient (y)
0.69
0.685
0.675
0.67
0.665
0.660
Activity coefficient (y)
0.445
0.405
0.395
Activity coefficient (y)
0.255
0.20
0.05
0.86
0.85
0.845
0.835
0.83
0.82
0.815
0.81
0.52
0.50
0.485
0.465
0.455
0.1
0.245
0.18
0.16
0.83
0.82
0.10
0.048
0.81
0.805 0.755
0.80
0.75
0.80
0.79
0.775
0.77
0.76
0.45
0.425
0.405
0.38
0.445 0.355
0.37
0.18
0.115
0.095
0.065
0.021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide hand writtin solution....arrow_forward16 A sample of gdd sulfide was found to contain. 24.54g of gold & 5.99 g this sample has a molar mass of 980,24 9/mol. of sulfur. In addition, a) fmpirical formula of sulI fide of gold sample b) Molecular formula of sulfide of gold samplearrow_forward22 problem pleasearrow_forward
- Which molecular formulas are also empirical formulas?SELECT ALL THAT APPLY. THERE IS MORE THAN ONE. C2H4 C5H5N5O C8H10N4O2 K2SO4 C6H6 Na2C2O4 C3H8 C6H12O6arrow_forwardreally need help with thisarrow_forwardTwo samples of solids have similar reactivity with acids and similar densitiesTheir masses and volumeshoweverare not at all similar Is possible that these are the same substance?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY