
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

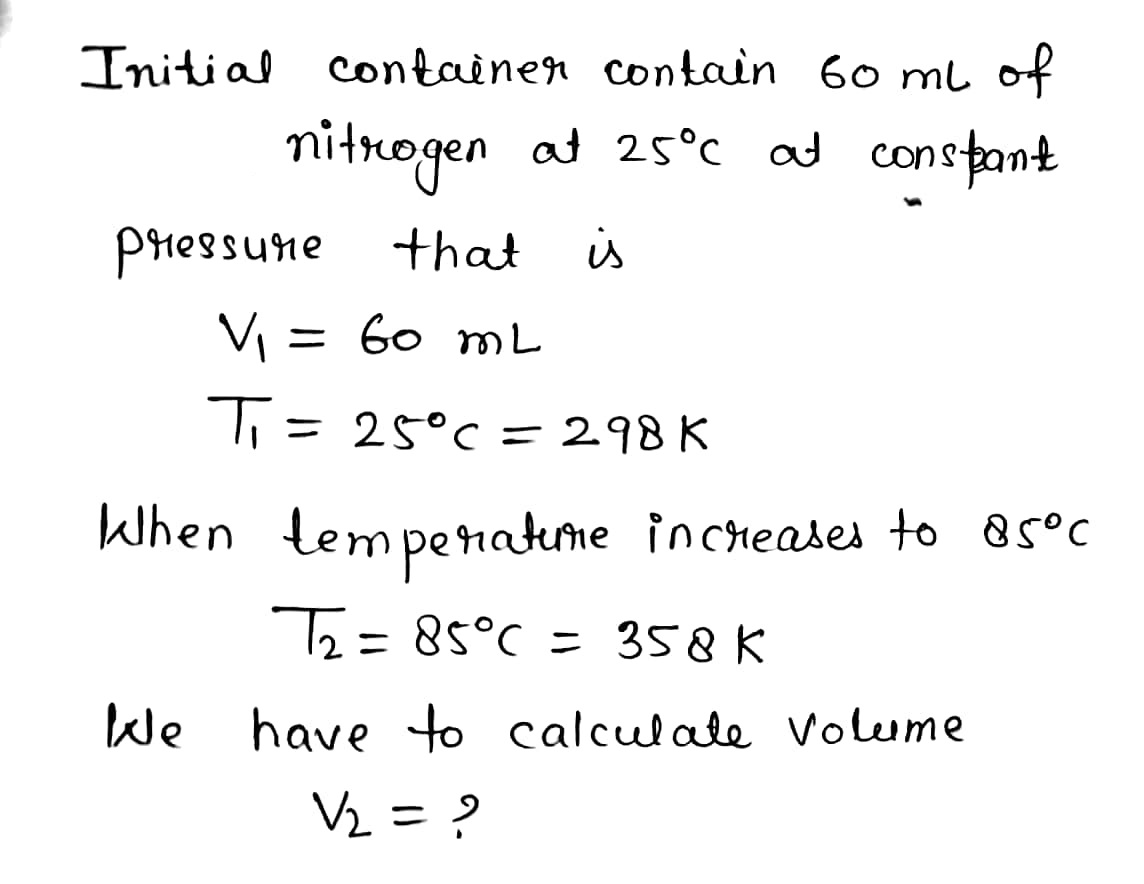
Transcribed Image Text:1. A container holds 60.0 mL of nitrogen at 25° C and at a constant pressure.
Find its volume if the temperature increases to 85° C?
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An arctic weather balloon is filled with 3.57 L of helium gas inside a prep shed. The temperature inside the shed is 7. °C. The balloon is then taken outside, where the temperature is -44. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits.arrow_forwardtakt Values if needed for this question. What is the total volume of gaseous products formed when 84.0 liters of bromine trifluoride react completely according to the following reaction? (All gases are at the same temperature and pressure.) bromine trifluoride(g) → bromine (g) + fluorine(g) Volume= Larrow_forwardSelect the most appropriate term for each relationship. You may use each choice once, more than once, or not at all. Volume is/has moles. Moles is/has temperature. Pressure is/has temperature. 1. proportional to 2. inversely proportional to Pressure is/has moles. 3. has no relationship to Pressure is/has volume. Volume is/has temperature. > 1001 Aarrow_forward
- What volume of carbon dioxide is produced when 130 liters of carbon monoxide react according to the following reaction? (All gases are at the same temperature and pressure.) carbon monoxide(g) + oxygen(g) ==> carbon dioxide(g)arrow_forwardA sample of oxygen gas initially at 311 K311 K was heated to 365 K.365 K. If the volume of the oxygen gas sample at 365 K365 K is 937.9 mL,937.9 mL, what was its volume at 311 K?arrow_forwardWhat is the new volume of a 1.75L sample of nitrogen that is heated from 25 C to 300 C?arrow_forward
- A ballon of 30.0 C has a volume of 222 mL. And the temp is increased to 52.1 Celsius and pressure remains constant what will the new volume be in mLarrow_forwardA 61.4 L volume of methane gas is heated from 16°C to 100°C at constant pressure. What is the final volume of the gas in L? (Do not include units in your answer. If you round during your calculation make sure to keep at least 3 decimal places. Report your answer to 1 decimal placearrow_forwardA sample of Nitrogen gas occupies a volume of 31.9 ml at 86.8°C. What temperature in °C will it need to occupy a volume of 73 ml?arrow_forward
- if the temperature in al paso is 306.15 k, the temperature in seattle is 295.15 k, and the temperature in salt lake city is 301.15 k what would be the volume of a ballon in seattle (1L) once it reached salt lake city. What about when it reached el paso?arrow_forwardts Name: 2. The Hindenburg airship used hydrogen gas instead of helium which, due to hydrogen's flammability, eventually resulted in tragedy. Assuming SATP, what was the density (in s/L) of hydrogen gas inside the Hindenburg?arrow_forwardIf there is a rigid container that contains helium has a volume of 1 L at 25 Kelvin. What would the volume be if the temperature was increased to 600oC ? ( need to use kelvin )arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY