
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
all one question, fill in the blank draw products
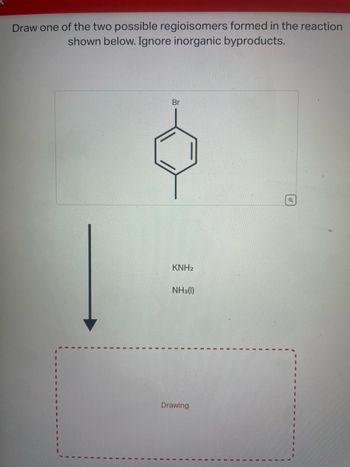
Transcribed Image Text:Draw one of the two possible regioisomers formed in the reaction
shown below. Ignore inorganic byproducts.
Br
KNH2
NH3(1)
Drawing
Q
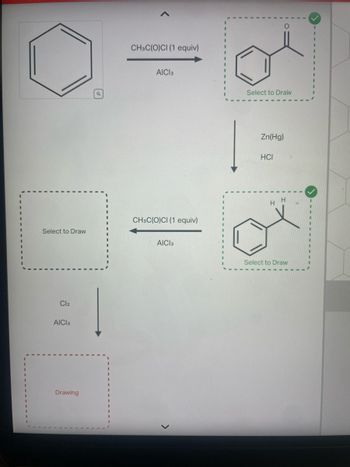
Transcribed Image Text:Select to Draw
Cl2
AICI 3
Drawing
CH3C(O)CI (1 equiv)
AICI 3
CH3C(O)CI (1 equiv)
AICI 3
Select to Draw
O
Zn(Hg)
HCI
H
H
Select to Draw
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the reaction, do not leave any fractions, don't leave anything blank, then answer the questions about the reaction Blank 1. Fill in the blank, read surrounding text. Hg3(PO3)2 + Blank 2. Fill in the blank, read surrounding text. Zn → Blank 3. Fill in the blank, read surrounding text. Hg + Blank 4. Fill in the blank, read surrounding text. Zn3(PO3)2 This is an _______ reaction. Is this a redox reaction? (yes/no) ______ , because Hg+2 is reduced to Hg0, and because Zn0 is ________ to Zn+2. Will the reaction happen as it is written? (yes/no) _______ , because the Activity Series tells us that Hg is more ________ than Zn. The reverse reaction would not happen.arrow_forwardUse the compound to write out the productsarrow_forwardCO2(g) + 2NH3(!) → CO(NH2)2(s) + H2O(1) This reaction models how urea fertilizer is produced from carbon dioxide and ammonia, with water as a byproduct. This is a chemical engineering process, and a urea factory is shown in this image:arrow_forward
- ( Problem Set #16 - predicting products of reactants 1. Complete and balance the following reactions - assume they all go a. solid cuprous chlorate plus heat b. solid stannic carbonate plus heat c. aluminum metal plus bromine liquid d. hydrogen gas plus nitrogen gas e. potassium metal plus sulfur powder solid magnesium oxide plus water f. g. potassium metal plus water h. solid sodium sulfide plus heat zinc metal plus oxygen gas solid potassium carbonate plus heat i. j. k. solid ferric chlorate plus heat 1. solid sodium chromate tetrahydrate plus heat m. solid ferric oxide plus water n. solid ferric oxide plus heat o. silver nitrate solution plus barium chloride solution p. sodium arsenate solution plus barium chloride solution q. potassium phosphate solution plus calcium nitrate solution r. phosphoric acid solution plus magnesium hydroxide s. lead metal plus silver nitrate solution t. chlorine gas plus sodium bromide solution u. C₂H₁6 plus oxygen gas v. Barium metal plus nitrogen gas…arrow_forwardLook at the first image for the equation and the information to do the two asked questions. Pls help ASAP. Pls do both of the asked questions I BEG.arrow_forwardWittig Reaction: Calculate the theoretical and percent yields of ethyl cinnamate. Use 0.15grams as the experimental yield. Show all steps and calculations.arrow_forward
- Using the data provided Mass of salicylic acid used: 4.0 g Mass of crude product: 5.8g Mass of pure product: 4.3 g Find the following and show all calculations: 1) Theoreticl yield of product 2) percent yield of crude product 3) percent yield of pure product *note this experiment used a reaction of 4.0g (0.030 mole) of salicylic acid and 6.0g (0.051 mole) of acetic anhydridearrow_forwardWhich generalized equation represents a double replacement reaction? a- AB--->A+B b-AB+CD--->AD+CD c- A+B--->AB d-A+BC--->B+ACarrow_forwardCharlie is balancing an equation. She has identified the atoms and counted the number of each in the reactants and products. What would Charlie adjust to make the number of atoms in the reactants the same as the number of atoms in the products? coefficients O subscripts O superscripts O parentheses Submit Next Save and Exit Mark this and return Sign outarrow_forward
- What is the product theoretical yield(g)? What is the percent yield(%)?arrow_forwardThe mass of the product formed in an experiment is theoretical yield? True or false?arrow_forwardHOMEWORK Balancing Equations 47. Balance the following equations: (a) CaC2(s) + H,O(l) Ca(OH)2(s) + C,H2(g) (b) (NH4)2Cr2O-(s) Cr2O3(s) + N2(g) + H2O(g) (c) CH;NH2(g) + O2(g) CO2(g) + N2(g) + H2O(g) 48. Balance the following equations: (a) CH12O6 (s) + O2(g)→ CO, (g) + H2O (1) (b) XeF4(g) + H2O (1) Xe (g) + O2(g) + HF (g) (c) NaCl (s) + H;O(g) + SO2(g) + O2 (g)- NazSO4 (s) + HCI (g) 15arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY