
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
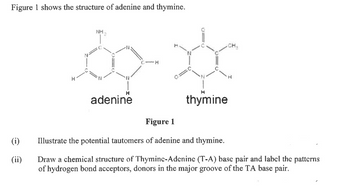
Transcribed Image Text:Figure 1 shows the structure of adenine and thymine.
(i)
(ii)
NH
adenine
C-H
thymine
Figure 1
Illustrate the potential tautomers of adenine and thymine.
Draw a chemical structure of Thyminc-Adenine (T-A) base pair and label the patterns
of hydrogen bond acceptors, donors in the major groove of the TA base pair.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- 6) Proteins can be modified by phosphorylation, which adds a phosphate group to the hydroxyl group of serine, threonine, or tyrosine residues. The R-group for phosphoserine is shown at right. A) The image below is an Isoelectric focusing strip that shows the unphosphorylated protein-of-interest (in blue). To which side of the unphosphorylated protein would you expect to see the phosphorylated protein? (Draw an arrow to indicate direction). Briefly justify your answer. un-phosphorylatable: Low pH Justify: B) To study the effects of phosphorylation, researchers often mutate a Ser/Thr to appear as though it is always phosphorylated or never phosphorylated at a particular site. What amino acid substitution should you use to preserve similar dimensions as Ser (or Thr) but make the side chain appear to be: constitutively (always) phosphorylated: Justify: Ser or Thr → O O=P-O O I CH₂ Ser or Thr➜ Phosphoserine High pHarrow_forwardDraw the structural formula of the oligopeptide if the amino acids are arginine, glutamine, glycine, methionine and glutamic acid considering the first amino acid is the N-terminusarrow_forwardHemoglobin is considered to be a tetrameric complex with a 64 kDa (α β)2. When attempting to purify hemoglobin, we must first purify the α and β monomers (about 16 kDa each) to prepare the tetramer. This is formed from the dimer intermediate: 2 α + 2 β -> 2 αβ -> (α β)2. The graph given represents a size-exclusion chromatogram after the refolding of the hemoglobin tetramer Using the size-exclusion chromatogram given, 1. Draw an SDS-Page Gel with a reducing agent such as BME using the three peaks listed on the graph.arrow_forward
- Proline is one of the amino acids in the "special" groups of sidechains. One reason for this is the dramatic impact it can have on secondary structure. a) draw the lewis structure for a tripeptide with the sequence Ala-Pro-Ala b) Proline residues are rarely found in alpha helices - in fact, they are often referred to as helix-disrupting amino acids. Thinking about intermolecular interactions, provide an explanation for why proline might disrupt alpha-helices. c) Proline also has a similar effect on beta-sheets, and is rarely found in the middle of beta sheets. Thinking about noncovalent interactions, provide an explanation for why proline might disrupt beta-sheets.arrow_forward(A) Give the polypeptide translation of the RNA sequence below. 5’-AUGGAAAUCAAAGUCAACCUUGAGUUUAGA-3’ (B) Write the chemical structure of the polypeptide sequence you determined in part (a) (C) Given the chemical structure of the polypeptide sequence you have written in part (b), answer True or False for each of the 5 statements below. (1) At least one of the amino acids in the sequence can undergo phosphorylation (2) The sequence looks like it could form a β turn (3) The sequence looks like it could form a β strand with one surface facing the interior of the protein and the other surface exposed to water (4) At least one of the amino acids in the polypeptide sequence can undergo oxidation to form a disulfide bridge to another polypeptide (5) The sequence looks like it could form an α helix that would be part of a coiled coil structure within a proteinarrow_forwardDraw the molecular formula of the covalently modifi ed histone side chain of acetyllysine. How does this modifi cation alter the chemical properties of the side chain?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON