
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
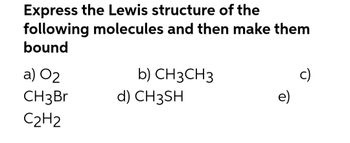
Transcribed Image Text:Express the Lewis structure of the
following molecules and then make them
bound
a) 02
CH3 Br
C2H2
b) CH3CH3
d) CH3SH
e)
c)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q8. How many σ and л bonds and unbonded electron pair(s) are present in the central chlorine atom of the chlorate ion [ClO3] in its Lewis structure with least formal charge? A) 3σ and 2л bonds and one unbonded electron pair. B) 3σ and Ол bonds and one unbonded electron pair. C) 4σ and Ол bonds and two unbonded electron pairs. D) 4σ and 2 bonds and one unbonded electron pair. E) 3σ and 3л bonds and one unbonded electron pair.arrow_forward1. Write a Lewis structure for each of the following: a) CHC13 b) SeBr2 c) NS+arrow_forwardFor the following 3 molecule / ions, XO3-, (X is a hyper-valent element in 5A group) IF 03 1) calculate the total number of valence electrons (show your work) 2) draw the Lewis Structure; if resonance exists, draw the resonance structures. 3) determine # electron domains, and # lone electron pairs on the central atoms 4) determine electron-pair geometry, molecular geometry (shape), hybrodization of the central atom. 5) determine bond angle, molecular polarity.arrow_forward
- Which of the following compounds does NOT exhibit covalent (molecular) bonding? A) CO₂ B) CO C) CoCl₂ D) HCOOH E) Na₂CO,arrow_forward3. Lewis structures of two chemical species are shown below. For each, give (i) the formal charge on each atom and (ii) the overall charge on each species. Structure a Structure b :F :O-CFo H- Atom 1 Atom 2 Atom 3 Atom 4 Atom 5 Хе Oa Ob Fa Fb H Cl Oa Ob Ос రీరేజిిarrow_forwardPlease don't provide handwritten solution ....arrow_forward
- Determine the number of valence electrons in SO₃ and then draw the corresponding Lewis structure (with minimized formal charges). A) 24 B) 20 C) 25 D) 28 E) 21arrow_forwardDetermine the number of valence electrons in (CH₃C(O)CN) and then draw the corresponding Lewis structure.arrow_forwardWrite the Lewis dot STRUCTURE for EACH of the following compounds.You MUST show the process! Note: NO double bonds are in ANY of the compounds.a) XeF4b) SeCl3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY