
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Explain the General Features of Dehydration in Acid ?
Expert Solution
arrow_forward
Step 1
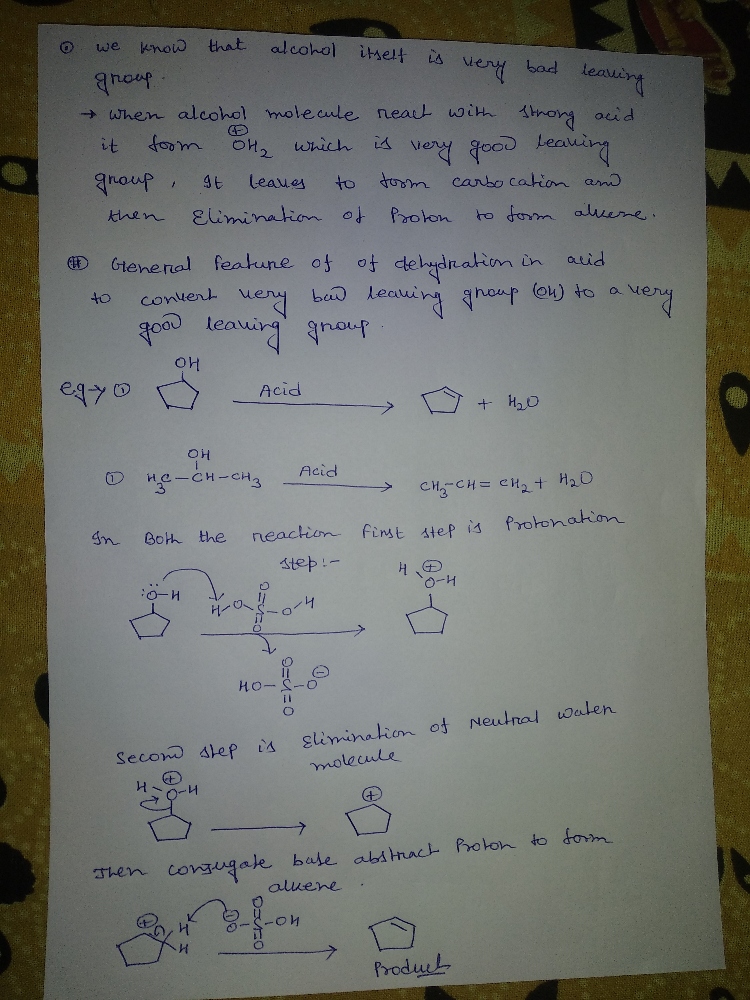
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is an oxide of nitrogen that serves as an anesthetic and analgesic.arrow_forward7) Draw the lactone formed by the following hydroxy carboxylic acid (Hint: Esterification reaction takes place.). سلل ОН OHarrow_forwardComplete the following chemical reaction by showing the structural formulas of all products The esterification of butanoic acid and 1-propanol Thank you!arrow_forward
- Complete the following chemical reaction by showing the structural formulas of all products The oxidation of 2-butanol. Thank you!arrow_forwardDraw out an appropriate chemical structure for the name provided. N-methoxyacetamidearrow_forwardWhy are some physicians are careless when prescribing benzodiazepines for patients suffering from severe anixety?arrow_forward
- Testosterone is a steroid. Predict the solubility of this structure in water.arrow_forwardCERCLA is one of the acts which has established the regulations for hazardous waste. CERCLA relates to: a toxicity of hazardous waste b disposal of hazardous waste c hazardous waste transportation d currently generated hazardous waste e clean up and past practices of hazardous wastearrow_forwardA hydrogen bond is a special case of which intermolecular force? Provide some examples of functional groups that are capable of participating in hydrogen bonding.arrow_forward
- Please do not give solution in image format thankuarrow_forwardwhy do amides boil at higher temperatures than hydrocarbons or ethers of similar molecular weightsarrow_forwardWrite the equilibrium-constant, Kp, expression for the reaction A(g) + 4 B(1) = 3 C(g) + D(g) Answer Bank Kp = 3Pc PA 4 P3 P Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY