
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
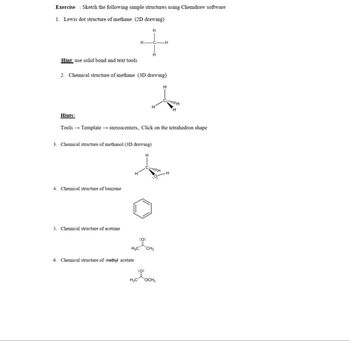
Transcribed Image Text:Exercise Sketch the following simple structures using Chemdraw software
1. Lewis dot structure of methane (2D drawing)
Hint: use solid bond and text tools.
Hints:
H
2. Chemical structure of methane (3D drawing)
3. Chemical structure of methanol (3D drawing)
4. Chemical structure of benzene
5. Chemical structure of acetone
6. Chemical structure of methyl acetate
Tools →→ Template → stereocenters, Click on the tetrahedron shape
H
H
:0:
H
:0:
H3C CH3
WITH
-H
H3C OCH3
H
CH
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Notes April In this exercise we have nitrogen, water, carbonate, carbon tetra chloride, ammonium, carbon monoxide, dinitrogen monoxide, nitrate, chlorite, phosphate, NF3, F2, SO42- and OCL2 !! Conclusion All of the compounds in this exercise are what kind of compound? Explain why this is important to VSEPR. Conclusion Explain why it is important to consider both VSEPR shape and bond polarity to determine the overall polarity of a molecule. Use the HF (hydrogen fluoride) and CO₂ (carbon dioxide) as examples.arrow_forwardI need helparrow_forwardO GENERAL CHEMISTRY REVIEW Writing Lewis structures for an expanded valence shell central.. Draw the Lewis structure for the sulfur tetrafluoride (SF) molecule. C Explanation Check 62021 McGraw-Hill Educati MacBook Pro esc G Search or type URL #3 2$ & 2 3 6 IIarrow_forward
- Which of the following molecules would have resonance structures? (Hint: you may want to draw Lewis structures for each) CO32- NH4+ H2O O3 A) NH4+ H2O and O3 B) CO32- and NH4+ C) CO32- NH4+ and H2O D) O3 only E) H2O only F) None of them G) All of themarrow_forwardPart A Write the Lewis structure for SO, where the central sulfur atom obeys the octet rule. Do not include any charges on your drawing. Show only one drawing (do not include any additional resonance structures). More Submit Request Answerarrow_forwardCheck all that are true for writing Lewis structures of polyatomic ions. Electrons are added to the total for a positive ion electrons are subtracted from the total for a positive ion electrons are added to the total for a negative ion electrons are subtracted from the total for a negative ionearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY