
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Write the balanced chemical equation
Convert reagent quantities into moles.
Find the amount of product that would be produced by each reagent
Chapter
Calculate the mass of product from the amount of limiting reagent.
What mass of the non-limiting reagent is in excess?
Calculate amount required for reaction, and subtract that from
amount added to reaction.
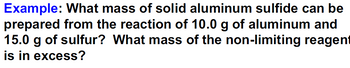
Transcribed Image Text:Example: What mass of solid aluminum sulfide can be
prepared from the reaction of 10.0 g of aluminum and
15.0 g of sulfur? What mass of the non-limiting reagent
is in excess?
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Actual yield is the calculated amount of product that is possible to obtain from a reaction. True/false?arrow_forwardPlease don't provide handwriting solutionarrow_forwardWhich of the following statements is not true but balancing chemical equation a. subscription and reactants must be conserved in the products b. coefficients are used to balance the atoms on both sides c. the law of conservation of matter must be followed d. phases (S,L,G,AQ) are often shown for each compound but I'm not critical to balancing an equation e. all of the above statements are truearrow_forward
- at t. Request to Withdr. U Forms | Office of t. KB Viewing Your Aca. Have Changes in. Schola Question 2.c of 8 Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. Step 2b: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al O, from the complete reaction of 201 grams Fe,0 according to the following balanced chemical equation: 2 Al(s) + Fe,O,(s) Al O,(s) + 2 Fe(s) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 201 101.96 1.26 128 256 159.70 257 315 2.52 64.2 g/mol AlO, mol AlO g/mol Fe,O, mol Fe O, g Fe,O, g Al,Oarrow_forwardWhich of the following is not a true statement concerning limiting reagent and excess reagent? A) The amount of product obtained is determined by the limiting reagent. B) A balanced equation is necessary to determine which reactant is the limiting. C) Some of the excess reagent is left over after the reaction is complete. D) Adding more of the limiting reagent to the reaction chamber will produce more products. E) The reactant that has smallest given mass is the limiting reagent. A В C O Earrow_forwardmatch the folowing terms. one answer will not be used. Left Side: limiting reactant actual yield theoretical yield percent yield mole ratio Right Side (match) maximum amount of product that could produced relationship between two different components of a balanced equation the reaction component that is consumed entirely the reaction component that is present in excess the actual yield divided by the theoretical yield 100 times amount of product physically produced from a reactionarrow_forward
- Write the balanced equation for the following situation. In addition, list the reaction type. You must tell the amount of every substance that remains in the container at the end of the reaction. Assume that all reactions go to completion. If only stoichiometry, tell how much excess reactant is used. Reaction Type:a. Combination Reactionb. Decomposition Reactionc. Single Displacement / THIS IS ONE TYPE OF Oxidation Reduction Reaction d. Precipitation Reactione. Gaseous Reactionf. Neutralization Reactiong. Combustion Reaction 61.802 cg of nitrogen gas is reacted with 61.802 cg of hydrogen gas to form ammoniaarrow_forward1. Calculate the theoretical yield (in grams) of salicylic acid from the given starting amount of methyl salicylate. Show the calculation with units. Given: 4.0 mL of methyl salicylate into a 250 Erlenmeyer flask for the conversion of methyl salicylate to salicylate acid synthesis reactionarrow_forwardHow many moles of C are formed upon completed reaction of 2 mol B according to generic chemical reaction A+B---->Carrow_forward
- Enter the formula of the missing substance (Y) in the balanced equation below. Do not give the coefficient. Mg + 2HCI -- MgCl2 + Y BALANCING CHEMICAL EQUATIONS INT-T03/S02 Enter the formula of the missing substance (Y) in the balanced equation below. Do not give the coefficient. 16Y + S88K2S FORMING EQUATIONS INT-T01/S03 Enter an unbalanced chemical equation that summarizes this statement: the reaction of solid silicon and solid octasulfur produces solid silicon disulfide.arrow_forwardWrite the balanced equation for the following situation. List the reaction type.Tell the amounts of every substance that remains in the container at the end of the reaction. Assume that all reactions go to completion. If only stoichiometry, tell how much of the excess reactant is used!!!! Reaction Type:a. Combination Reactionb. Decomposition Reactionc. Single Displacement / THIS IS ONE TYPE OF Oxidation Reduction Reaction d. Precipitation Reactione. Gaseous Reactionf. Neutralization Reactiong. Combustion Reaction Follow the format used in the image. 5.92 g of sodium oxalate is reacted with 5.92 of calcium chloridearrow_forwardCalculate th number of g of carbon dioxide produced by heating 90.0 g of strontium carbonate. balance equation (including the physical states). Strontium carbonate decomposes upon heating to strontium oxide and carbon dioxide. Enter and Be ure o nswer ll parts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY