
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Solve this whole problem and include the interpolation of the same table.
Transcribed Image Text:PDF *Elementary Principles of Chemic X PDF Elementary Principles of Chemica X +
49°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDF Drive%20).pdf
Draw
(T) Read aloud
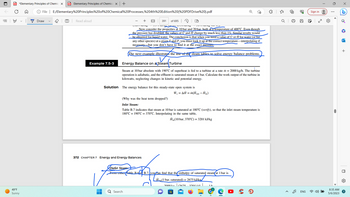
Example 7.5-3
Solution
391 of 695
Energy Balance on a Steam
JESTITIS
JUTERIIS TULIT, JORING
JUTE Iing for
Now consider the properties at 10 bar and 20 bar. both at a temperature of 400°C. Even though
the pressure has doubled, the values of Û and Ĥ change by much less than 1%. Similar results would
be obtained for liquid water. The conclusion is that when you need a value of Û or Ĥ for water (or for
any other species) at a given T and P, you must look it up at the correct temperature-interpolating if
necessary but you don't have to find it at the exact pressure.
The next example illustrates the use of the steam tables to solve energy balance problems.
eam Tur
Turbine
Steam at 10 bar absolute with 190°C of superheat is fed to a turbine at a rate m = 2000 kg/h. The turbine
operation is adiabatic, and the effluent is saturated steam at 1 bar. Calculate the work output of the turbine in
kilowatts, neglecting changes in kinetic and potential energy.
372 CHAPTER 7 Energy and Energy Balances
OD
The energy balance for this steady-state open system is
Q Search
(Why was the heat term dropped?)
Inlet Steam:
Table B.7 indicates that steam at 10 bar is saturated at 180°C (verify), so that the inlet steam temperature is
180°C + 190°C = 370°C. Interpolating in the same table,
Ĥin (10 bar, 370°C) = 3201 kJ/kg
W₁ = AH = m(Ĥout - Ĥin)
Outlet Steam:
From either Fable B. 6/0 B.7, you can find that the enthalpy of saturated steam at 1 bar is
Hout (1 bar, saturated) = 2675 kJ/kg/
2000 (2675 32014-1
H
1h
{
J
ENG
Sign in
100
8:35 AM
5/6/2023
•
la
+
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Please I want a detailed explanation of the energy method rule placed in the first line and how these numbers appeared to us. I want a detailed explanation please urgent .arrow_forwardDerive Newton's Raphson Formula from scratch using Taylor seriesarrow_forward56.0 mL of two different liquids are poured through a funnel with a narrow exit tube, and the time for all of the liquid to flow through is recorded. Here are some results: time to flow through funnel trial Liquid X Liquid Y 1.60 s 3.62 s 1.52 s 3.44 s 1.44 s 3.56 s Note: the two liquids have the same density. Which statement below is true about these liquids based only on the data in the table? O The intermolecular forces in liquid X are stronger than those in liquid Y. The boiling point of liquid X is higher than the boiling point of liquid Y. The viscosity of liquid X is greater than the viscosity of liquid Y. The surface tension of liquid X is greater than the surface tension of liquid Y. The viscosity of liquid X is less than the viscosity of liquid Y. 3.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY