
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
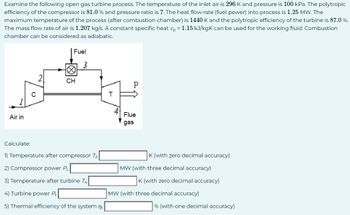
Transcribed Image Text:Examine the following open gas turbine process. The temperature of the inlet air is 296 K and pressure is 100 kPa. The polytropic
efficiency of the compressor is 81.0% and pressure ratio is 7. The heat flow rate (fuel power) into process is 1.25 MW. The
maximum temperature of the process (after combustion chamber) is 1440 K and the polytropic efficiency of the turbine is 87.0 %.
The mass flow rate of air is 1.207 kg/s. A constant specific heat cp = 1.15 kJ/kgk can be used for the working fluid. Combustion
chamber can be considered as adiabatic.
Air in
Calculate:
O
Fuel
∞
CH
3
1) Temperature after compressor T2
2) Compressor power Pc
3) Temperature after turbine T4
4) Turbine power Pt
5) Thermal efficiency of the system nt
H
P
Flue
gas
K (with zero decimal accuracy)
MW (with three decimal accuracy)
K (with zero decimal accuracy)
MW (with three decimal accuracy)
% (with one decimal accuracy)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 31 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Required information A turbojet is flying with a velocity of 900 ft/s at an altitude of 20,000 ft, where the ambient conditions are 7 psia and 10°F. The pressure ratio across the compressor is 13, and the temperature at the turbine inlet is 2320 R. Assume ideal operation for all components and constant specific heats for air at room temperature. The properties of air at room temperature are Cp = 0.24 Btu/lbm-R and k = 1.4. Determine the propulsive efficiency. The propulsive efficiency is 45.79 %.arrow_forwardAssuming a compression ignition engine having a cut-off ratio of 2 and a compression ratio of 20. Before the compression stroke, air is at 100kPa, 20°C and a volume of 500 cm³. Determine (in kPa) the mean effective pressure of the cyclearrow_forwardTemperature after the heat addition process = K Thermal efficiency =% Mean effective pressure = kpaarrow_forward
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY