
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
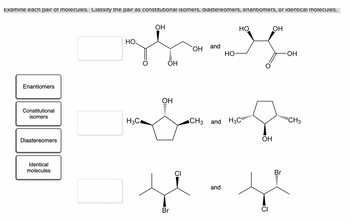
Transcribed Image Text:Examine each pair of molecules. Classify the pair as constitutional isomers, diastereomers, enantiomers, or identical molecules.
Enantiomers
Constitutional
isomers
Diastereomers
Identical
molecules
НО.
H3C,
ОН
ОН
ОН
ОН
Br
and
но-
НО
CH3 and H3C
CI
and
нан
ОН
CI
ОН
Br
-ОН
СН3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Basic discussion
VIEW Step 2: Rules for assigning R/S
VIEW Step 3: Finding R/S for the first sets of compound
VIEW Step 4: The relationship for first pair of compound
VIEW Step 5: The relationship for second pair of compound
VIEW Step 6: The relationship for third pair of compound
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the blank. Constitutional Isomer? Conformational Isomer? Enantiomer? Diastereomer?arrow_forward4. Isomers and Stereocenters ( (a) Assign each set of molecules as constitutional isomers, conformational isomers, enantiomers, diastereomers, or identical. Please write the full name for the type. Molecules Type (b) H₂Ca HgC.. CH3 OCH₂ CH3 af OCH, SP H₂C**** H₂C H₂C NGOCH, H.C_OCKS OCH₂ CH₂ H₁C HC H*** CI H H CI Br H3C CH₂ Br (d) E) (0) For each of the following molecules, write the number of stereocenters and circle the center of any stereocenters. (i) (ii) H₂C (онт (eg 5) (b) -CH3 Isegna CH3 on mots eno H₂C CH3arrow_forwardWhat term best describes the relationship between the structures below? Br CI Br Diastereomers Conformational isomers Identical Constitutional isomers Enantiomers CIarrow_forward
- What are the differences between stereoisomers, constituional isomers, conformational isomers, and configuration isomers. Please show examplesarrow_forwardPlease don't provide handwriting solutionarrow_forwardDefine the relationship between the molecule pairs shown below. Choose from the following relationships: Different Molecules Constitutional Isomers Conformational Isomers Geometric Isomers Enantiomers Diastereomers Write the relationship in the space provided. Relationship (A) Identical Br: Conformational isomers AND (В) I dentical enantiomers :Br: AND он (C) OH он Constilulional isomers AND --arrow_forward
- Compare the following conformers of 1-fluoropropan-1-ol. Match each conformer to their corresponding relative energy. Recall, the more stable the conformer the lower the energy. OH CH3 'F 2. HOH 2nd Highest Energy H 2nd Lowest Energy H3C 3. H. ОН Lowest Energy H 'F Highest Energy ČH3 4. H3C, OH 'F 1. >arrow_forwardWhat is the definition of the term “stereoisomer”?Isomers that differ with respect to the order in which the atoms are connectedIsomers which differ in the arrangement of atoms in spaceIsomers that differ in molar massIsomers that differ by bond rotations onlyIsomers that have different boiling pointsarrow_forwardMa Two disubstituted cyclohexane molecules are depicted. Classify the pair as the same compound, enantiomers, diastereomers, constitutional isomers, or not isomeric. CI CI and CI The compounds are: the same compound not isomeric diastereomers enantiomers constitutional isomers CIarrow_forward
- 2. H3CO H3CO HO 1. Consider the molecule shown below. [1] Draw the two chair conformations for the compound. [2] Identify which of the two chair conformations is more stable and explain why. H3CO Jonoto NH₂ How are the following molecules related? (exactly the same, completely different, constitutional isomers, enantiomers, or diastereomers). OH OH OCH3 HO“ OH H3CO H3CO is content is protected and may not be shared, uploaded of ributed OCH3 HO,,, OH O... "OCH3arrow_forwardThe following reaction forms a 50:50 mixture of two compounds. What is their relationship? Options: Conformers (different conformations) Structural isomers Enantiomers Diastereomersarrow_forward(a) Classify each of the following pairs of structures as constitutional isomers, conformational isomers, configurational isomers or identical. Br and Br Br NH, CH, HO но H,C. ii. and CH, CH, Vi. and он Ph CH, он CH, Ph iv. andarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER